A kind of solid preparation and preparation method thereof
A technology of solid preparations and adhesives, which can be used in pill delivery, pharmaceutical formulations, and medical preparations of non-active ingredients. It can solve problems such as restrictions on the viscosity and properties of active ingredients, and achieve shortened research and development time, reduced weight and size, and The effect of improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
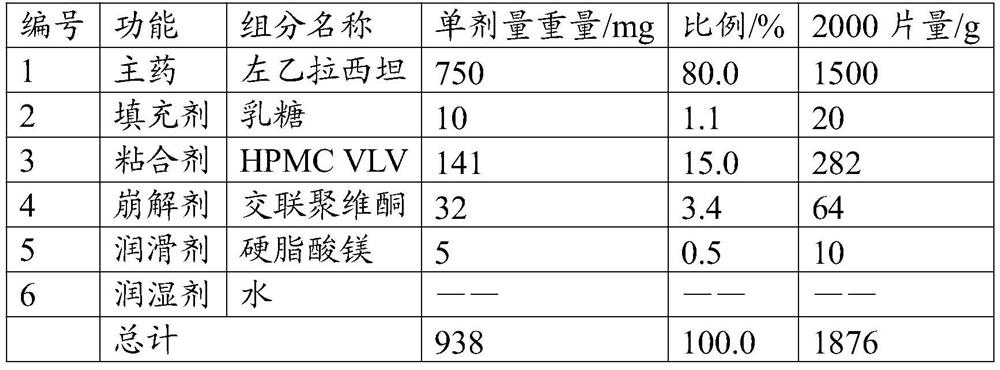
[0039] The present embodiment provides a solid preparation with high drug loading, and its prescription is as follows:
[0040]
[0041] Its preparation method is as follows:
[0042] (1) Pulverize the main ingredient so that 60% of the weight passes through a 200 mesh sieve, and the weight of more than 60 mesh sieve is less than 2%. Combine with the sieved filler and disintegrant, and pre-mix for 5 minutes in a high-shear granulator to obtain a pre-mixed material.
[0043] (2) The binder is formulated into an aqueous solution with a mass concentration of 25%, added to the premixed material, granulated by a high-shear granulator, dried at 50°C for 10 minutes, and granulated to obtain a dry intermediate, wetted The agent water is eventually removed.
[0044] (3) Add a lubricant to the dry intermediate and mix for 5 minutes to obtain granules.
[0045] (4) Compressing the prepared granules with a tablet machine to obtain the solid preparation with high drug loading.
[00...
Embodiment 2
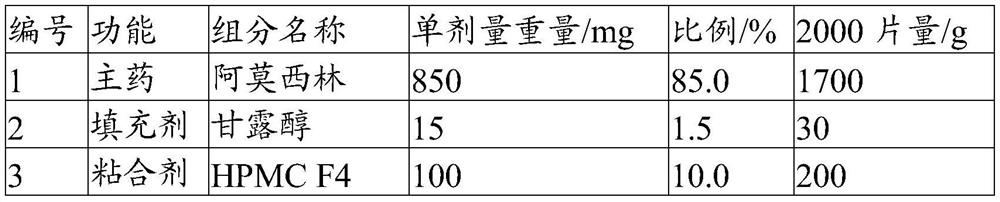
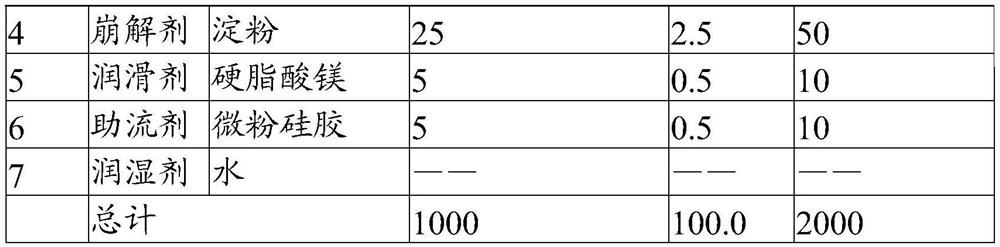
[0049] The present embodiment provides a solid preparation with high drug loading, and its prescription is as follows:
[0050]
[0051]
[0052] Its preparation method is as follows:
[0053] (1) Pulverize the main ingredient so that 60% of the weight passes through a 200 mesh sieve, and the weight of more than 60 mesh sieve is less than 2%. Combine with the sieved filler and disintegrant, and pre-mix for 5 minutes in a high-shear granulator to obtain a pre-mixed material.
[0054] (2) The binder is formulated into an aqueous solution with a mass concentration of 20%, added to the premixed material, granulated by a high-shear granulator, dried at 50°C for 8min, and granulated to obtain a dry intermediate, wetted The agent water is eventually removed.
[0055] (3) Add a lubricant and a glidant to the dry intermediate and mix for 5 minutes to obtain granules.
[0056] (4) Compressing the prepared granules with a tablet machine to obtain the solid preparation with high ...
Embodiment 3
[0060] The present embodiment provides a solid preparation with high drug loading, and its prescription is as follows:
[0061]
[0062] Its preparation method is as follows:
[0063] (1) Pulverize the main ingredient so that 60% of the weight passes through a 200 mesh sieve, and the weight of more than 60 mesh sieve is less than 2%. Combine with the sieved filler and disintegrant, and pre-mix for 5 minutes in a high-shear granulator to obtain a pre-mixed material.
[0064] (2) The binder is formulated into an aqueous solution with a mass concentration of 20%, added to the premixed material, granulated by a high-shear granulator, dried at 55°C for 4min, and granulated to obtain a dry intermediate, wetted The agent water is eventually removed.
[0065] (3) Add a lubricant and a glidant to the dry intermediate and mix for 5 minutes to obtain granules.
[0066] (4) Compressing the prepared granules with a tablet machine to obtain the solid preparation with high drug loading...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com