Enterokinase light chain mutant and application thereof
A technology of enterokinase and mutants, applied in the field of bioengineering, can solve the problems of unstable characteristics of enterokinase, difficulty in purification and preservation, application limitations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Embodiment 1, bovine enterokinase mutant
[0091] The amino acid sequence of the light chain of wild-type bovine enterokinase (EK) is as follows (SEQ ID NO: 1):
[0092]
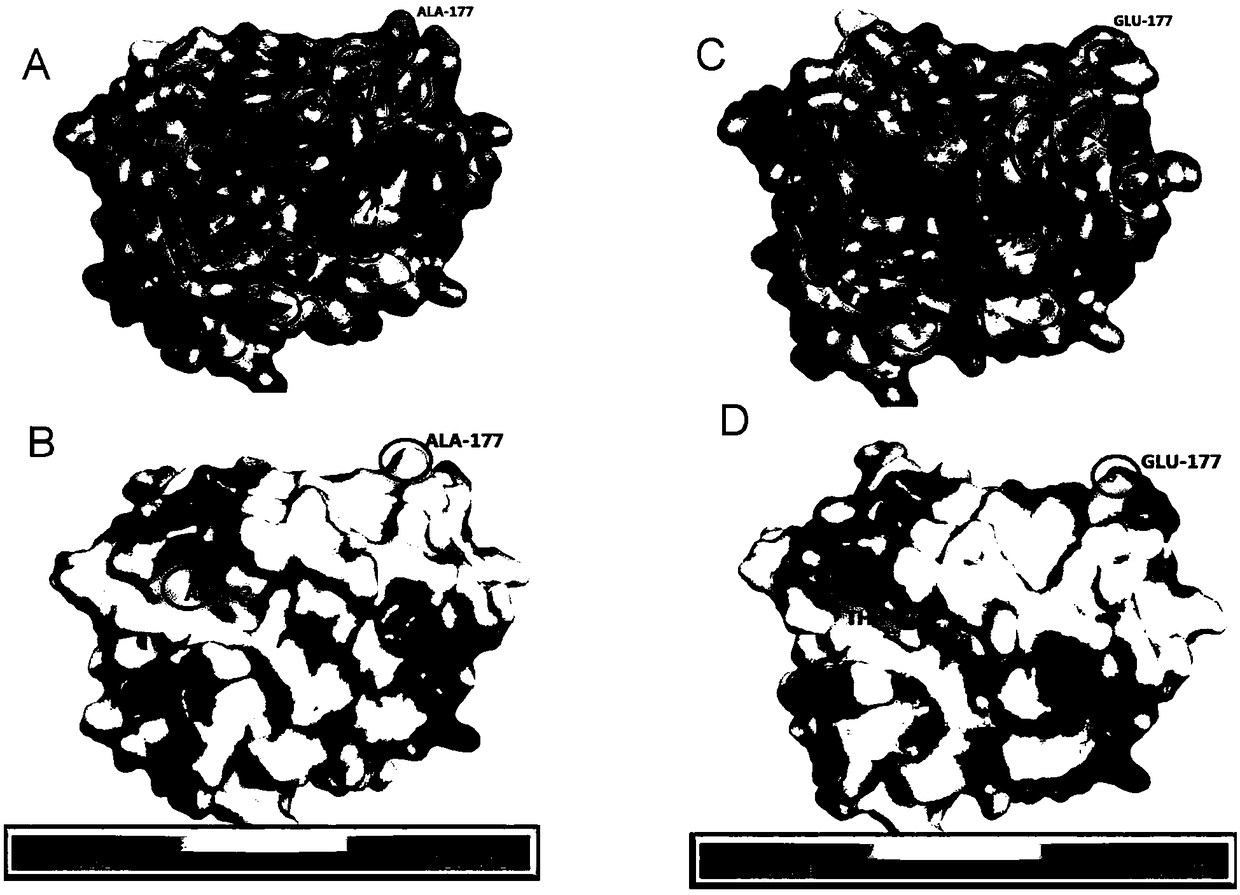
[0093] For each amino acid position of the EK light chain, the inventors have carried out a large number of analysis, comparison and experiments, and found that two hydrophobic amino acids Ala-62 and Ala-177 located on the surface of the three-dimensional structure of the protein, such as figure 1 , the R group of the amino acid is a hydrogen atom, which does not interact with other amino acid side chains in the protein structure.
[0094] The inventors mutated the amino acids at Ala-62 and Ala-177 to threonine and glutamic acid respectively, which can increase the hydrophilicity and local charge on the surface of EK protein, and can increase the stability of EK in solution It can also increase its renaturation rate.
[0095] At the same time, the inventor intends to carry out a mutation at Cys-1...
Embodiment 2
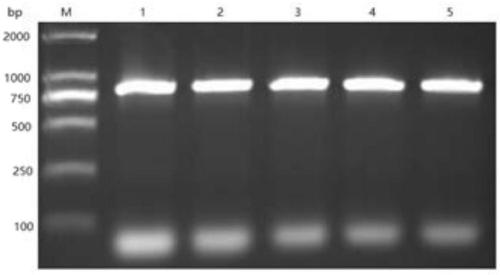
[0097] The PCR identification of embodiment 2, clone colony
[0098] Synthesizing the wild-type gene and its mutant gene, the mutant gene encodes a mutant polypeptide with mutations at the following sites on the basis of the wild-type amino acid sequence in Example 1: C112A, C112T, C112A-A62T-A177E, C112T-A62T-A177E.
[0099] Construct the synthetic mutant gene in the multi-cloning site of the expression plasmid pET28a, transform the synthetic recombinant plasmid into the expression strain BL21(DE3), smear the plate, culture at 37°C overnight, and pick a single colony with good growth on the transformation plate Into 3 mL of LB medium containing kana-resistant, incubate on a shaker at 37°C for 5-6 hours, and perform colony PCR when the bacterial solution is turbid and visible to the naked eye. Samples were added sequentially according to the colony PCR system.
[0100] PCR system: a total of 20 μL
[0101]
[0102] PCR conditions
[0103]
[0104] The colony PCR iden...
Embodiment 3
[0105] Embodiment 3, induced expression
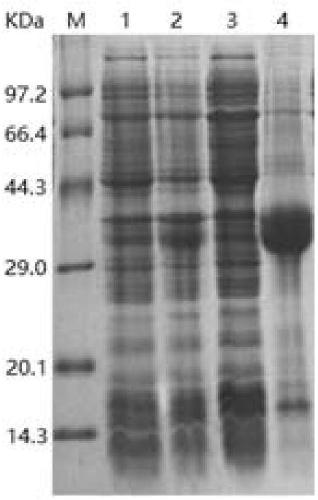
[0106] The bovine enterokinase light chain mutant C112T-A62T-A177E was induced and expressed with 1 mM IPTG.
[0107] Detected by SDS-PAGE protein electrophoresis, the band after induction was slightly concentrated at 30kDa compared with the band before induction, the supernatant band had no obvious change after ultrasonication, and the precipitated band was obviously thicker at 30kDa. Expressed as inclusion body expression. Such as image 3 (Taking the C112T-A62T-A177E mutant as an example).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com