Alk7 binding proteins and uses thereof
A technology for binding proteins and proteins, applied in the field of ALK7 binding proteins and their uses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0283] Preparation of ALK7-binding protein
[0284] In some embodiments, the ALK7 binding protein binds the extracellular domain of ALK7. In additional embodiments, the ALK7-binding protein is an anti-ALK7 antibody such as a full-length anti-ALK7 antibody or an ALK7-binding antibody fragment and variants and derivatives thereof.
[0285] ALK7 binding proteins can be readily prepared using known techniques. Monoclonal anti-ALK7 antibodies can be prepared using techniques known in the art, including hybridoma methods such as those described by Kohler and Milstein, Nature 256:495-497 (1975). Using the hybridoma approach, mice, hamsters, or other suitable host animals are immunized as described above to elicit lymphocytes to produce antibodies that specifically bind to the immunized antigen. Lymphocytes can also be immunized in vitro. Following immunization, lymphocytes are isolated and fused with an appropriate myeloma cell line to form hybridoma cells, which can then be selec...
Embodiment 1
[0404] Example 1. Selection, Characterization and Generation of ALK7 Binding Antibodies
[0405] Human IgG antibodies that bind ALK7 with high affinity were selected using a multi-round selection procedure, which is described in detail below.
[0406] Materials and methods
[0407] Proteins containing human ALK7-Fc were biotinylated using the EZ-Link Sulfur-NHS-Biotinylation kit from Pierce. Goat anti-human F(ab') 2 Kappa-FITC (LC-FITC), Extravidin-PE (EA-PE) and streptavidin-633 (SA-633) were obtained from Southern Biotech, Sigma and Molecular Probes, respectively. Streptavidin microbeads and MACS LC separation column were purchased from Miltenyi Biotec.
[0408] Experiments were performed at 25°C and 37°C using Biacore T100 / T200 biosensors (Biacore / GE Healthcare). ALK7 antibody was captured on a custom FAB chip. A series of concentrations of ALK7-Fc containing protein were injected over the flow cell at a flow rate of 50 μl / ml. To obtain kinetic rate constants, the cor...
Embodiment 2
[0420] Example 2. Characterization of ALK7-binding antibodies
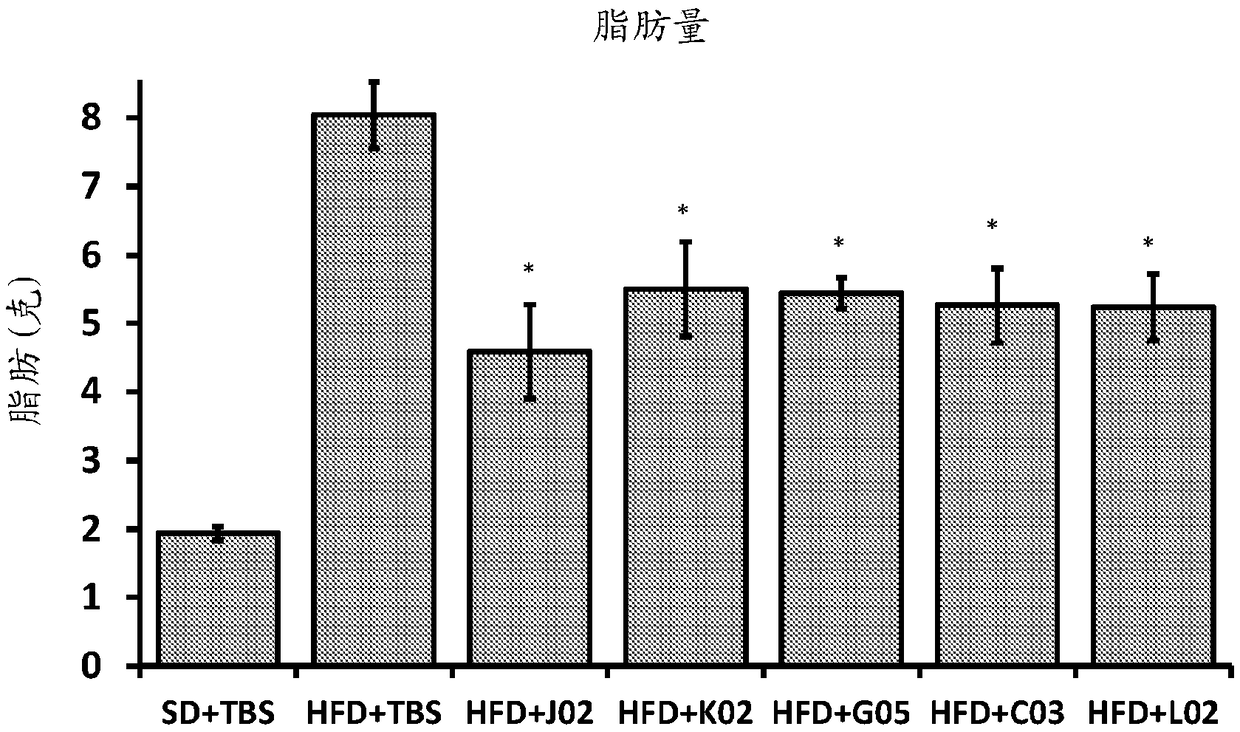
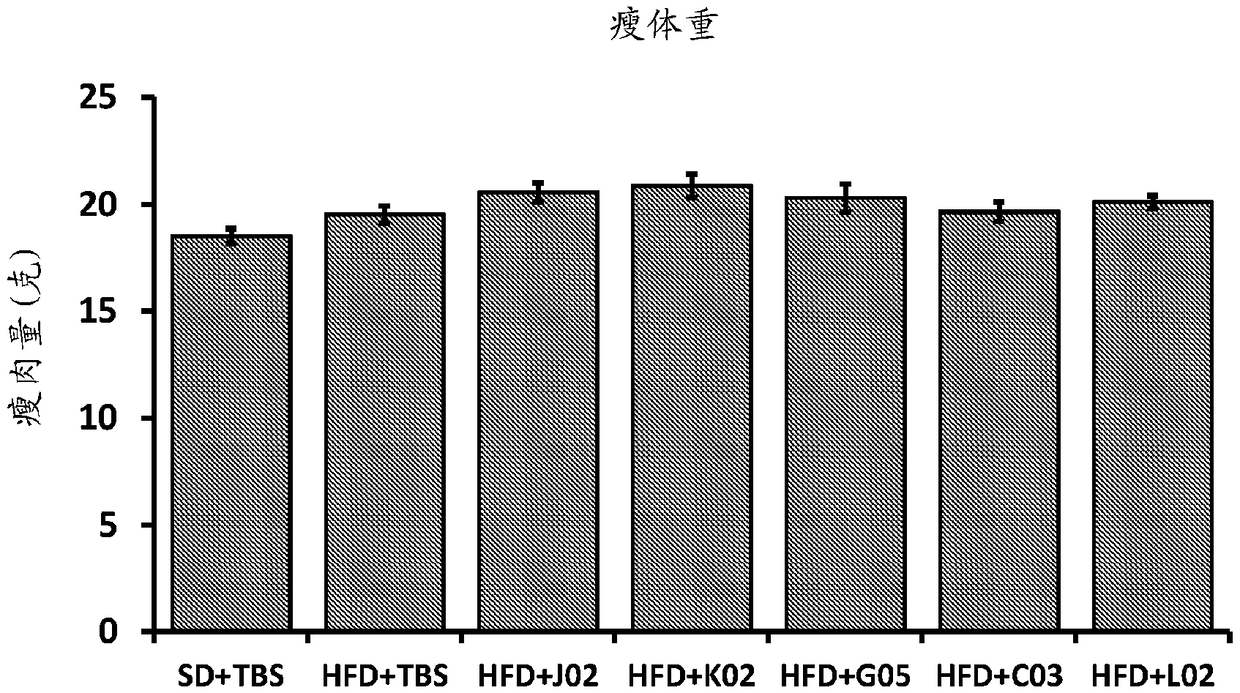
[0421] Exemplary ALK7-binding proteins generated according to the preceding examples were further characterized by sequence, SPR, and cell-based lipolysis inhibition assay analysis.
[0422] The sequences of exemplary ALK7-binding antibodies generated according to the methods described in Example 1 are presented in Table 1A (exemplary CDR sequences are underlined).
[0423] Table 1A : Exemplary ALK7 binding protein
[0424] G04
[0425] VH FR1
QVQLVQSGAEVKKPGSSVKVSCKASGGTFS (SEQ ID NO: 6)
VH CDRI
SYAIS (SEQ ID NO: 1)
VH FR2
WVRQAPGQGLEWMG (SEQ ID NO: 7)
VH CDR2
GIIPIFGTASYAQKFQG (SEQ ID NO: 2)
VH FR3
RVTITADESTSTAYMELSSLRSEDTAVYYCAR (SEQ ID NO: 8)
[0426]
[0427] C02
[0428]
[0429]
[0430] D04
[0431]
[0432] H03
[0433]
[0434]
[0435] Table 1B : Additional Exemplary ALK7 Binding Proteins
[0436] J01
[0437] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com