Coxsackie virus CVA16 virulent strain CVA16-B6-714 and application thereof
A Coxsackie virus, CVA16 technology, applied in the field of medicine and biology, can solve the problem of less research on the virulence site and attenuation mechanism of CVA16
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, induction and preparation of CVA16-B6 virulent strain
[0041] 1. Establishment of the first generation of mouse-adapted strain (CVA16-B)
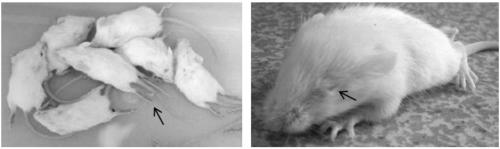
[0042] Dilute the CVA16-P4 strain to 1 × 10 6 , 1×10 5 and 1×10 4 CCID 50 / ml at three dilutions, respectively inoculate three groups of 1-day-old Kunming mice by intracranial route (20 μl / rat), 8 rats in each group. At the same time, a negative control group was established, and DMEM virus maintenance solution was injected intracranially, and the observation period was 14 days. The mice were taken back at the time point when the mice became seriously ill, the surface was disinfected with 75% alcohol, and the brains of the mice were taken out under aseptic conditions. Add DMEM virus maintenance solution to grind the mouse brain, prepare a 10% (W / V) homogenate, centrifuge at 3,000×g for 10 minutes at 4°C, take the supernatant, and inoculate 100 μl to Vero cell culture expansion for the first generation, which is mo...
Embodiment 2
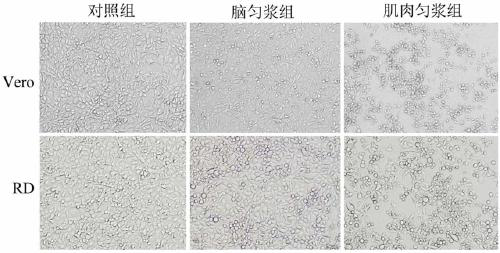
[0046] Embodiment 2, the toxicity detection of CVA16-B6 virulent strain
[0047] 1. Three consecutive plaque purifications ( figure 2 , 3 )
[0048] (1) 10-fold gradient dilution of the CVA16-B6 strain virus liquid by 10 -1 to 10 -6 Six dilutions.
[0049] (2) Take a six-well plate covered with a monolayer of Vero cells, discard the culture medium, wash the cell surface with PBS, add virus solutions of different dilutions into the six-well plate, 200 μl / well, repeat two wells for each dilution , placed at 37°C, 5% CO 2 Incubate for 1 h in the incubator.
[0050] (3) Take 1% agarose covering solution and add it to a six-well plate, 3ml / well, and place it at 37°C and 5% CO after the agarose solidifies. 2 Cultivate in the incubator for 3-5 days.
[0051] (4) Observe the six-well plate under an inverted microscope. When typical CVA16 plaques are observed, add 0.01% neutral red staining solution, 2ml / well, and place at 37°C, 5% CO 2 Stain overnight in the incubator.
[0...
Embodiment 3
[0066] Embodiment 3, the sequence determination of CVA16-B6-714 virulent strain
[0067] 1. Viral RNA extraction
[0068] (1) Take 140 μl of the harvested virus liquid, add 560 μl buffer AVL, mix with a vortex shaker for 15 seconds, let stand at room temperature for 10 minutes, and centrifuge briefly.
[0069] (2) Add 560 μl of absolute ethanol to the above mixture, mix with a vortex shaker for 15 seconds, and centrifuge briefly.
[0070] (3) Take 630 μl of the above mixture and carefully add it to the column, centrifuge at 6,000×g for 1 min, and discard the waste liquid.
[0071] (4) Repeat step (3).
[0072] (5) Carefully add 500 μl buffer AW1, centrifuge at 6,000×g for 1 min, and discard the waste.
[0073] (6) Carefully add 500 μl buffer AW2, centrifuge at 21,000×g for 3 minutes, and discard the waste.
[0074] (7) Put the column into a new 2ml collection tube and centrifuge at 26,000×g for 1min.
[0075] (8) Put the column into a new 1.5ml centrifuge tube, add 60μl d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com