Application of compound in preparing medicine for treating acute pulmonary embolism
A technology for pulmonary embolism and a compound is applied in the application field of the compound in the preparation of a medicine for treating acute pulmonary embolism, and can solve problems such as reports on the effect of pulmonary embolism that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
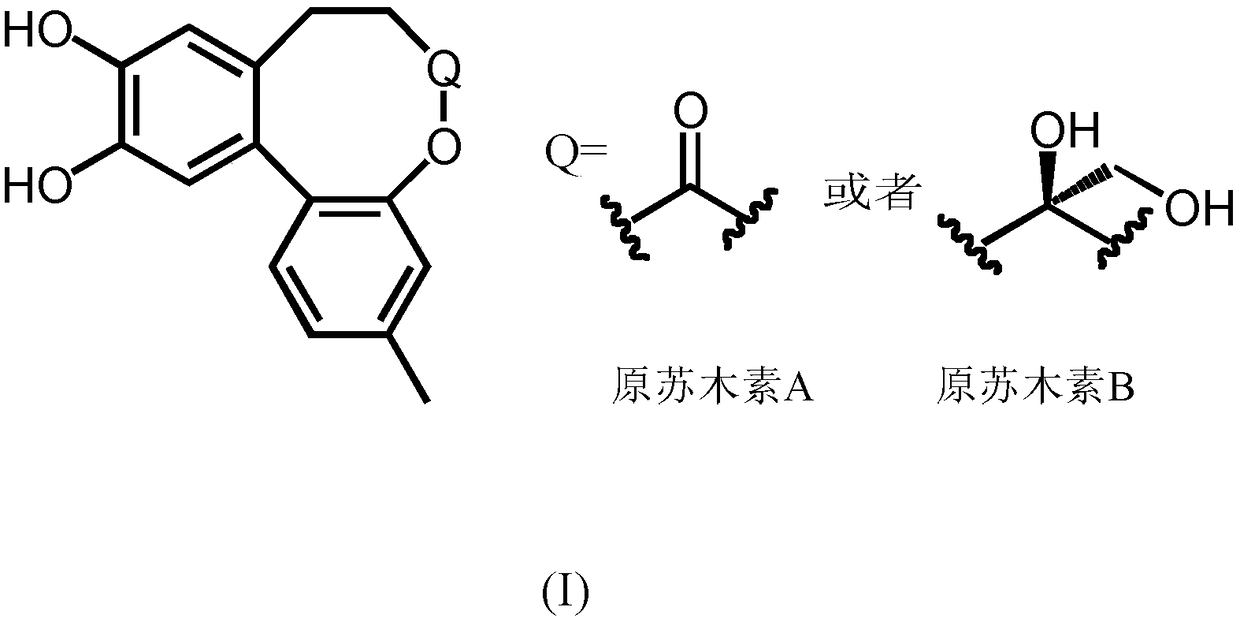
[0024] Example 1 Isolation and identification of prohematoxylin A
[0025] Hematoxylin dry heartwood (21kg), cut into small thin strips, reflux extraction with 8 times, 6 times, 6 times of 95% ethanol successively, 1 hour each time; the extract solution decompresses and recovers the solvent to obtain 95% ethanol extract. After evaporating the solvent until there is no alcohol smell, suspend in water, extract with petroleum ether, ethyl acetate, and n-butanol in sequence, and recover the solvents respectively to obtain 60 g of petroleum ether extract, 1400 g of ethyl acetate extract, and extract with n-butanol Material 360g.
[0026] Take 800g of ethyl acetate extract, and go through silica gel (100-200 mesh) column chromatography, chloroform-methanol=30:1, 20:1, 10:1, 5:1, 1:1 gradient elution, TLC comparison and combination A total of 14 fractions of Fr.A-Fr.N were obtained. Fraction Fr.E was subjected to silica gel (200-300 mesh) column chromatography, petroleum ether-et...
Embodiment 2
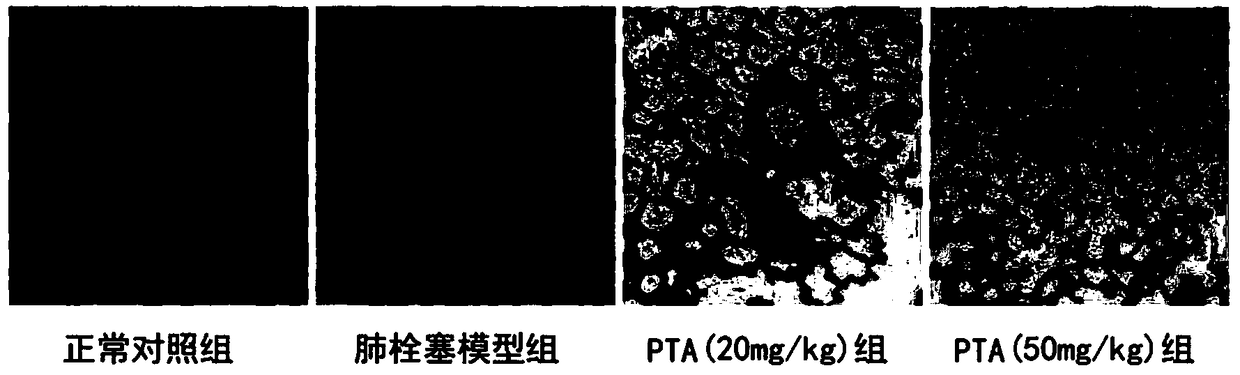
[0040] Example 2 Improvement effect of prohematoxylin A on lung index in rats with pulmonary infarction
[0041] 2.1 Test animals: SD rats, 8 weeks old, male, Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.
[0042] 2.2 Test drugs:
[0043] Protohematoxylin A (PTA) was dissolved in 0.5% CMCNa solution to prepare a 5 mg / mL drug solution.
[0044] 2.3 Grouping and handling of animals:
[0045] 40 SD rats, after one week of adaptive feeding, 8 in each group, were randomly divided into 5 groups: sham group (normal control group), model group (pulmonary infarction model group), PTA low-dose group (20mg / kg) , PTA high dose group (50mg / kg). The way of administration is intragastric administration.
[0046] Except for the normal control group, the animals in each group were subjected to the following operations:
[0047] One day before the operation and 40 minutes before the operation, the normal control group and the model group were given the same amount of nor...
Embodiment 3
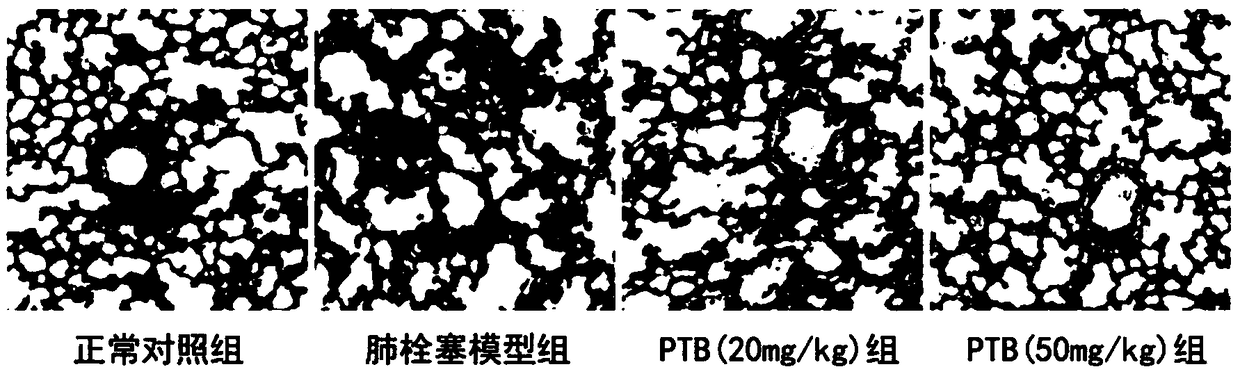
[0059] Example 3 Effect of Protohematoxylin A on Respiratory Frequency of Rats with Pulmonary Infarction
[0060] 3.1 Test animals: SD rats, 8 weeks old, male, Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.
[0061] 3.2 Test drugs:
[0062] Protohematoxylin A (PTA) was dissolved in 0.5% CMCNa solution to prepare a 5 mg / mL drug solution.
[0063] 3.3 Grouping and handling of animals:
[0064] 40 SD rats, after one week of adaptive feeding, 8 in each group, were randomly divided into 5 groups: sham group (normal control group), model group (pulmonary infarction model group), PTA low-dose group (20mg / kg) , PTA high dose group (50mg / kg). The way of administration is intragastric administration.
[0065] Except for the normal control group, the animals in each group were subjected to the following operations:
[0066] One day before the operation and 40 minutes before the operation, the normal control group and the model group were given the same amount of no...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com