Construction method and detection method of UPLC characteristic chromatogram of lophatherum gracile medicinal material
A construction method and a feature map technology, applied in the field of medicine, can solve the problems of inability to reflect the basic characteristics of traditional Chinese medicine decoction materials, and it is difficult to fully reflect the quality characteristics of pale bamboo leaves, so as to achieve good medicinal material quality, overcome durability deviation, and ensure the effect of quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
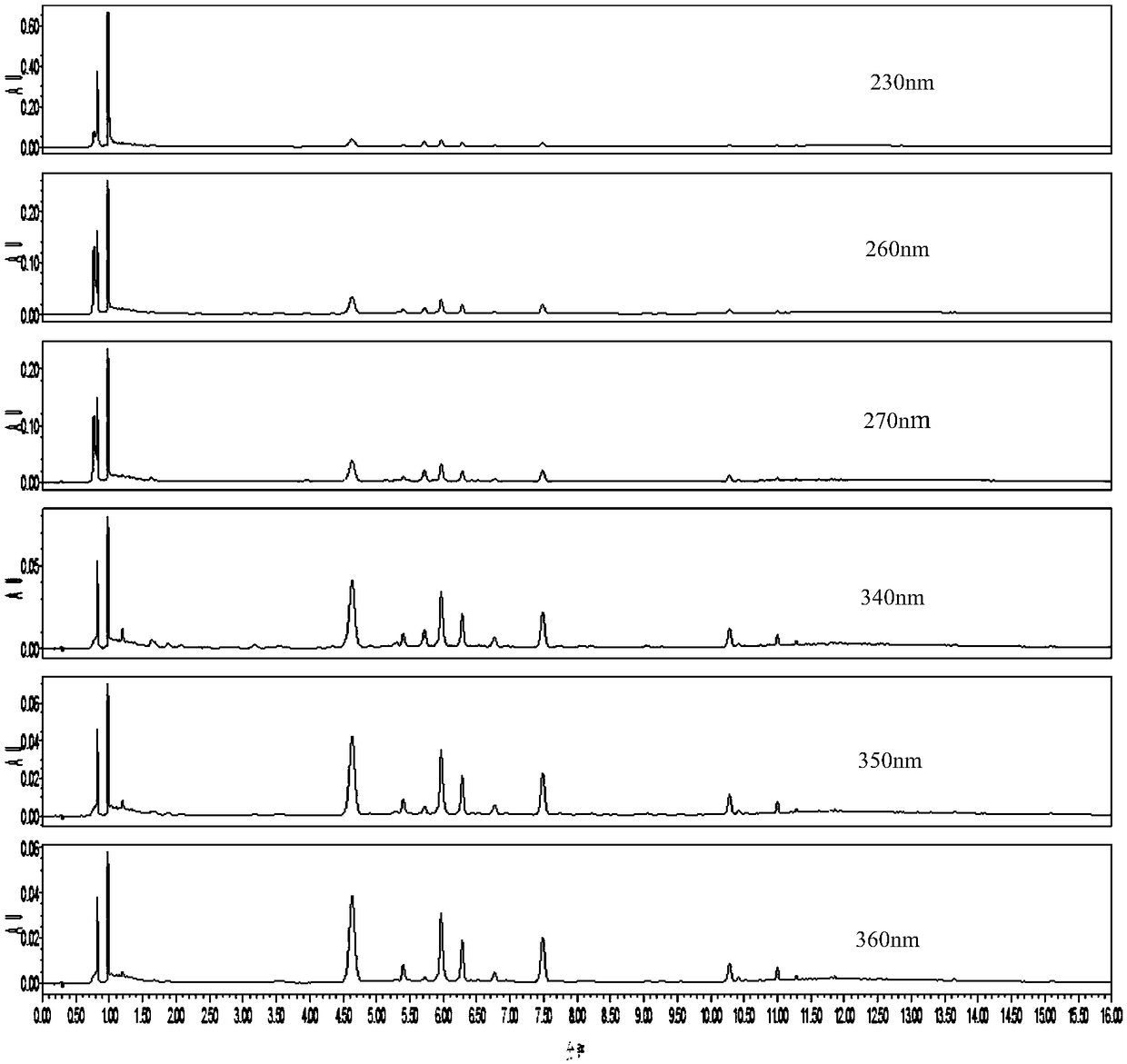
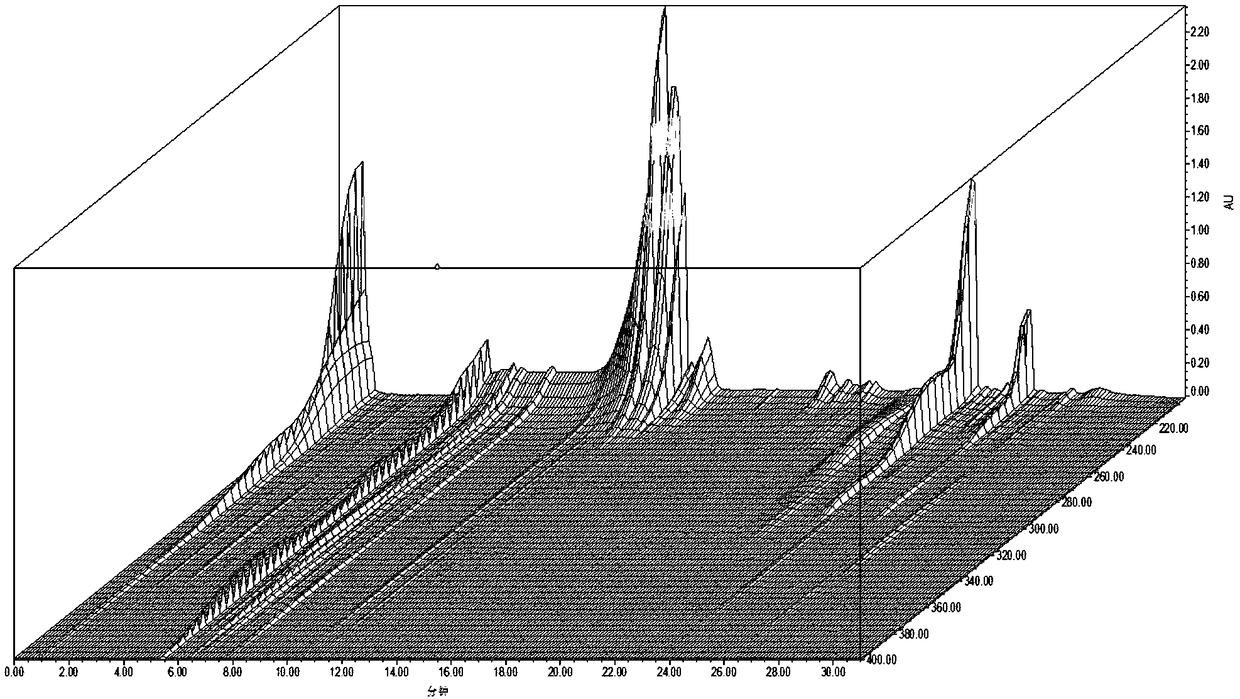
[0062] Example 1 The construction of the characteristic map of bamboo leaves medicinal material
[0063] 1. Instruments, reagents and reagents
[0064] The instruments are shown in Table 1, the reagents are shown in Table 2, and the reagents are shown in Table 3:
[0065] Table 1
[0066]
[0067] Table 2
[0068]
[0069] table 3
[0070]
[0071]
[0072] 2. Research object
[0073] In this embodiment, the medicinal materials of dandelion leaves come from 17 batches of samples from 7 authentic or major production areas with large dandelion leaf production in the country, as shown in Table 4 for details:
[0074] Table 4
[0075]
[0076] Remarks: The content of isoorientin is determined according to the content determination method under the item of "Hong Kong Chinese Medicinal Materia Medica" under the item of Elephant Bamboo.
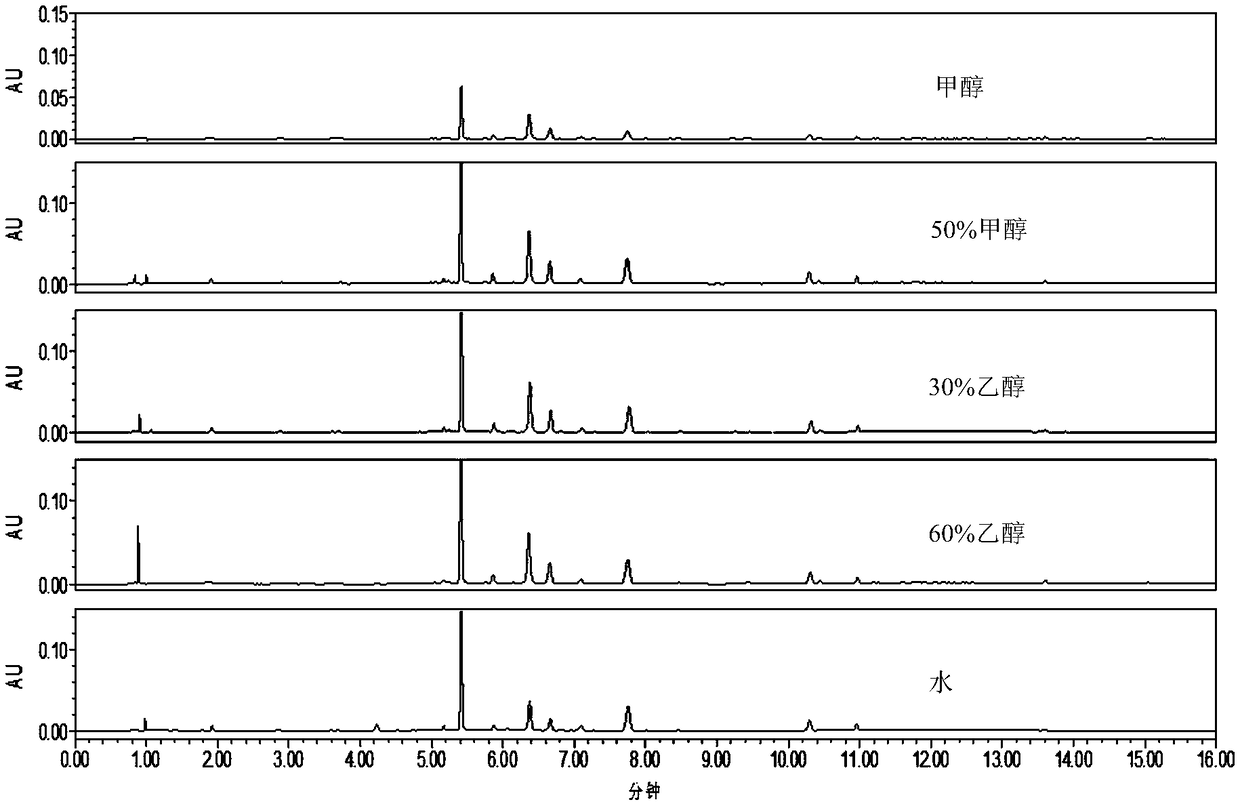
[0077] 3. Preparation of reference substance reference solution, reference medicinal material solution and standard decoction ...
Embodiment 2
[0157] The detection method of embodiment 2 light bamboo leaf medical material
[0158] The present embodiment provides a kind of detection method of light bamboo leaf medical material, and the steps are as follows:
[0159] (1) Chromatographic conditions
[0160] With 4.1 in embodiment 1.
[0161] (2) Preparation of reference substance reference substance solution
[0162] Same as 3 in Example 1.
[0163] (3) Preparation of the sample solution to be tested
[0164] Take 1.0g of the sample to be tested (batch number: DZY18, qualified according to the Chinese Pharmacopoeia), put it in a 50mL volumetric flask, add an appropriate amount of 60% (volume fraction) ethanol aqueous solution, ultrasonically treat (power 500W, frequency 40kHz) for 30 minutes, and extract solution, the extract was allowed to cool, and the volume was fixed to the mark with 60% (volume fraction) ethanol aqueous solution, shaken up, filtered, and the subsequent filtrate was taken as the sample solution to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass-to-charge ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com