Dual real-time fluorescence PCR primer group, kit and method for identifying Atlantic salmon and rainbow trout simultaneously
A real-time fluorescence and rainbow trout technology, applied in biochemical equipment and methods, recombinant DNA technology, microbial measurement/inspection, etc., can solve the problems of salmon aquatic product market supervision, long detection cycle, time-consuming and labor-intensive problems, and achieve enhanced The effect of market supervision, reduced testing cost, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 A dual real-time fluorescent PCR primer set and probe for simultaneous identification of Atlantic salmon and rainbow trout
[0042] The primer set of the present invention can simultaneously detect Atlantic salmon and rainbow trout. The primer set of the present invention includes F1 and R1, F2 and R2, and the base sequences thereof are as follows.
[0043] F1: 5'-agcagaactc agccagcct-3' (SEQ ID NO: 1),
[0044] R1: 5'-aaaggaggga gggagaagtc aa-3' (SEQ ID NO: 2),
[0045] P1: 5'-ccttctggga gatgacc-3' (SEQ ID NO: 3);
[0046] F2: 5'-accatttatta acataaaacc tccag-3' (SEQ ID NO: 4),
[0047]R2: 5'-gtaatgcctgctgccagga-3' (SEQ ID NO: 5),
[0048] P2: 5'-cgtttgagcc gtgcta-3' (SEQ ID NO: 6);
[0049] The above-mentioned F1 and R1 are Atlantic salmon-specific primers, and P1 is an Atlantic salmon-specific probe. The 5' end of P1 is modified by the fluorescent group FAM, and the 3' end of P1 is modified by the non-fluorescent quencher group MGB. F2 and R2 are rainb...
Embodiment 2
[0050] Example 2 A dual real-time fluorescent PCR method for simultaneous identification of Atlantic salmon and rainbow trout
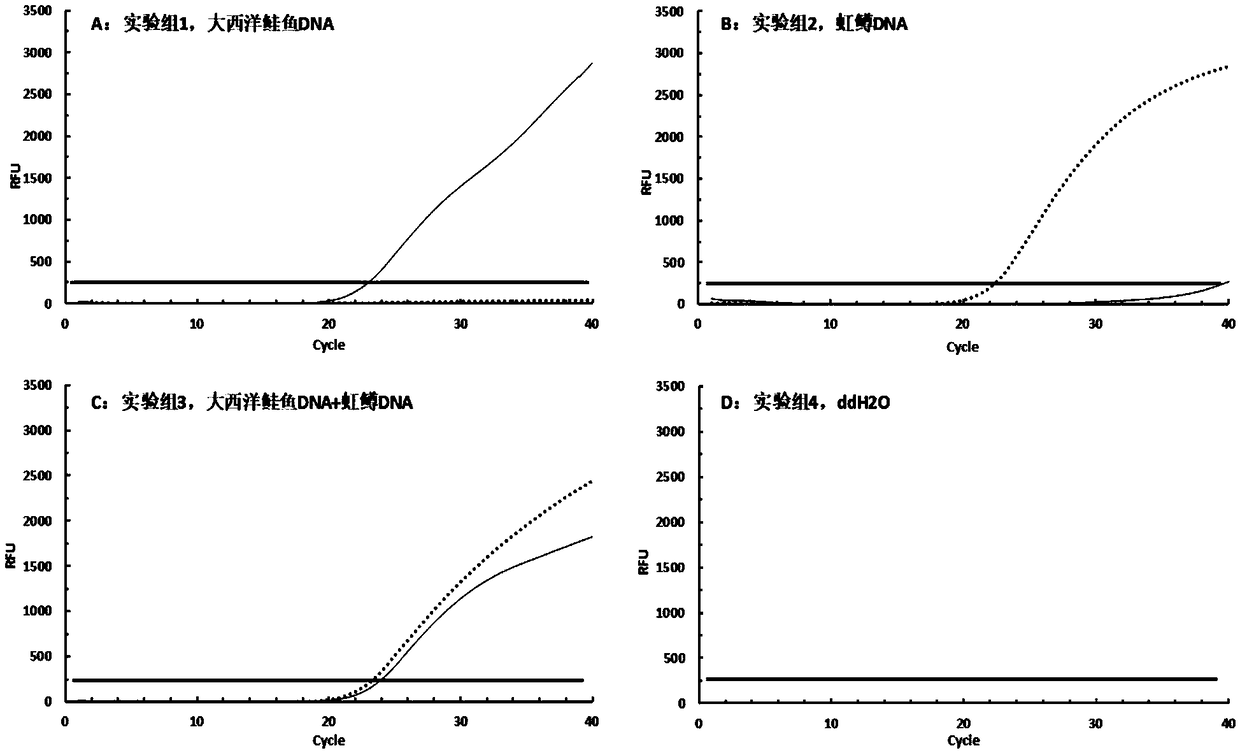
[0051] In this embodiment, four experimental groups are set. Add 50ng of Atlantic salmon DNA to the tube of experimental group 1, add 50ng of rainbow trout DNA to the tube of experimental group 2, add 25ng of Atlantic salmon DNA and 25ng of rainbow trout DNA to the tube of experimental group 3, add Add ddH in 4 2 O served as a negative control.
[0052] 1) Extract sample DNA; weigh the sample with sterile scissors and place it in a 2 mL sterile centrifuge tube, use a commercial animal tissue DNA extraction kit for DNA extraction, and use a nucleic acid protein analyzer to measure the concentration of the extracted DNA.
[0053] 2) Using DNA as a template, using the above-mentioned F1 and R1, F2 and R2 as primers, and P1 and P2 as probes, perform double real-time fluorescent PCR amplification;
[0054] Among them, the dual real-time fluorescent PCR ...
Embodiment 3
[0063] Embodiment 3 specific detection
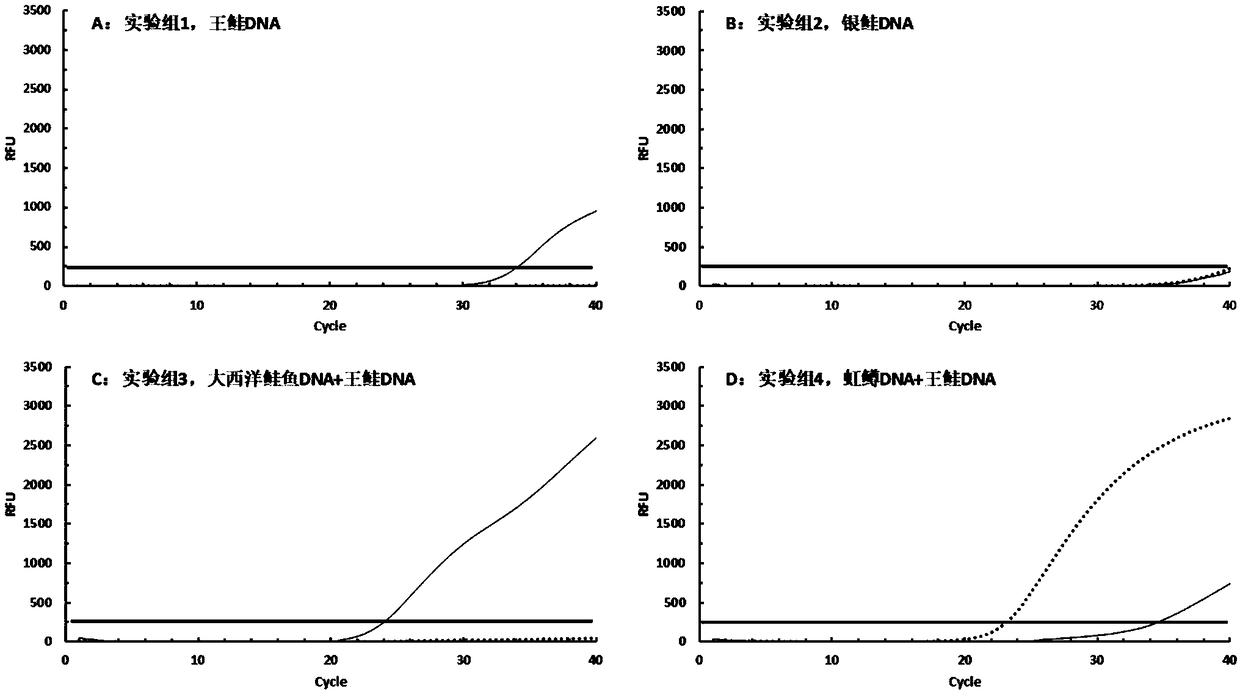
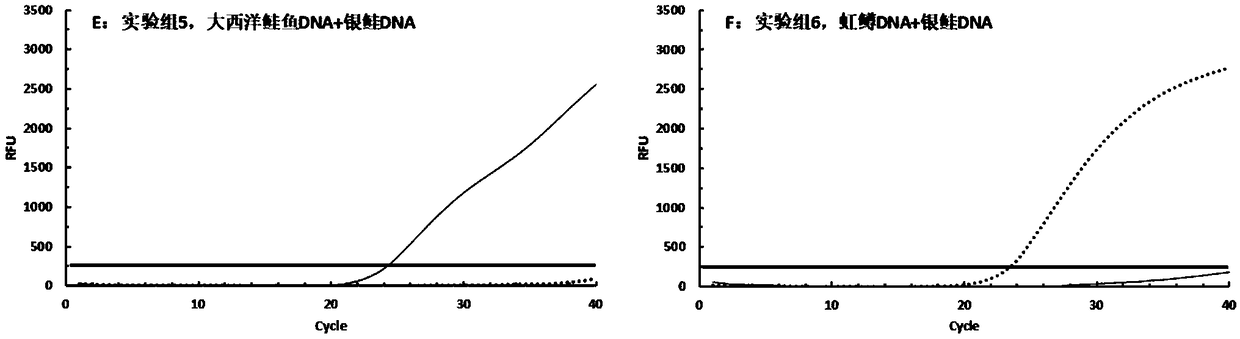
[0064] In this example, the DNA of king salmon (Oncorhynchus. Tshawytscha) and coho salmon (Oncorhynchus. Kisutch), which are relatively common in the salmon market in China, was used to verify the specificity of the primers. Set up six experimental groups. Add 50ng king salmon DNA to the tube of experimental group 1, add 50ng coho DNA to the tube of experimental group 2, add 25ng Atlantic salmon DNA and 25ng king salmon DNA to the tube of experimental group 3, and add 25ng DNA of king salmon to the tube of experimental group 3. Add 25ng rainbow trout DNA and 25ng king salmon DNA to tube 4, add Atlantic salmon DNA and 25ng coho DNA to tube 5, add rainbow trout DNA and 25ng coho DNA to tube 6 DNA.
[0065] 1) Extract sample DNA: Weigh the sample with sterile scissors and place it in a 2 mL sterile centrifuge tube, use a commercial animal tissue DNA extraction kit for DNA extraction, and use a nucleic acid protein analyzer to measure th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com