Preparation method of plasma human immunoglobulin
A human immunoglobulin and plasma technology, applied in the preparation methods of serum immunoglobulins, immunoglobulins, peptides, etc., can solve the problems of quality control of immunoglobulin finished products, and achieve the cost saving of gelation and the reduction of load. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
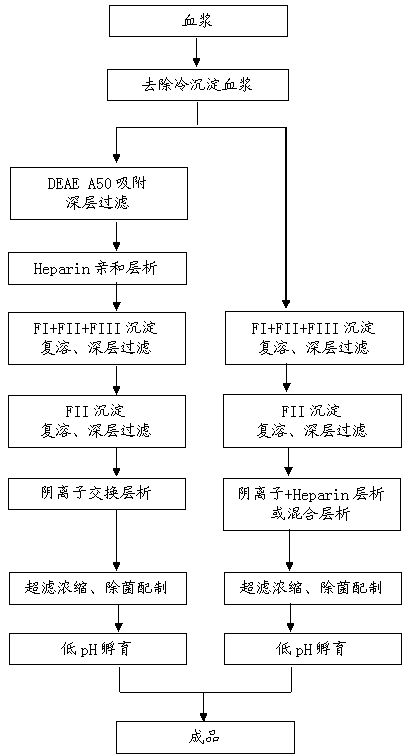
[0042] (1) Plasma to remove cryoprecipitate.
[0043] (2) Adsorption on DEAE Sephadex A50 gel. Collect the flow-through plasma and washing solution, which is the plasma after A50 adsorption. The eluate was used to prepare PCC product.
[0044] The Zeta Plus 60ZB adsorption material was washed with water for injection, and then washed with 100mM NaCl, 20mM NaCl 2 HPO 4 , pH6.5 solution cleaning, the dosage is 50L / m 2 ; After washing, drain and dry the adsorption material with compressed air, and then perform impurity adsorption on the plasma after A50 gel adsorption.
[0045] After the plasma was filtered through a 0.2 μm filter element, fixed-bed affinity chromatography was carried out through a chromatographic column packed with Capto Heparin affinity gel. The chromatographic column is treated with an equilibrium solution containing disodium hydrogen phosphate 10mmol / L, sodium chloride 0.3mol / L, and pH6.50, and the amount of each sample is controlled to be 30 column bed ...
Embodiment 2
[0056] (1) Plasma to remove cryoprecipitate.
[0057] (2) Plasma that has not been adsorbed by A50 gel directly enters the next step (3) for processing.
[0058] (3) Precipitation of FI+FII+FIII, adsorption of impurities, and reconstitution.
[0059] (4) FII precipitation, impurity adsorption, and redissolution.
[0060] (5) Use Zeta Plus 30ZB and 60ZB adsorption materials for overlapping use; use water for injection to clean the adsorption materials, and then use 5mM Na 2 HPO 4 , pH6.5 solution to clean the adsorption material, the dosage is 50L / m 2 ; After washing, drain the solution in the equipment and dry the adsorption material with compressed air, and then perform impurity adsorption on the supernatant after reconstitution of FII prepared from plasma that has not been adsorbed by A50 gel.
[0061] The supernatant after impurity adsorption, was filtered through a 0.2 μm filter element, and packed with Fractogel ® The chromatographic columns of EMDTMAE gel and Capto...
Embodiment 3
[0068] (1) Plasma to remove cryoprecipitate.
[0069] (2) Plasma that has not been adsorbed by A50 gel directly enters the next step (3) for processing.
[0070] (3) Precipitation of FI+FII+FIII, adsorption of impurities, and reconstitution.
[0071] (4) FII precipitation, impurity adsorption, and redissolution.
[0072] (5) Zeta Plus 30ZB and Zeta Plus 90ZB adsorption materials are used superimposed; wash with water for injection, and then use 15mM Na 2 HPO 4 , pH7.5 solution for cleaning, the dosage is 50L / m 2 ; After washing, drain the solution in the equipment and dry the adsorption material with compressed air, and then perform impurity adsorption on the supernatant after reconstitution of FII prepared from plasma that has not been adsorbed by A50 gel.
[0073] The supernatant after impurity adsorption was filtered through a 0.2 μm filter element, and fixed-bed chromatography was performed using a chromatographic column filled with DEAE Sepharose Fastflow gel. The ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com