Application of iron removal drug in preparation of H7N9 bird flue preventive drugs
A bird flu and drug technology, applied in the field of biomedicine, can solve the problems of weak iron ion scavenging effect and inability to remove iron ions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The present invention is further described below in conjunction with specific embodiment, but protection scope of the present invention is not limited thereto:
[0031] Desferrioxamine: desferrioxamine B, a chelating agent, mainly complexes with trivalent ions and trivalent aluminum ions to form complexes, and the complex formation constants are 1031 and 1025 respectively; but for divalent ions such as ferrous (Fe++ ), copper (Cu++), zinc (Zn++) and calcium (Ca++), etc. have very low affinity, and their complex formation constants are 1014 or lower. Chelation is performed on a 1:1 molar basis.
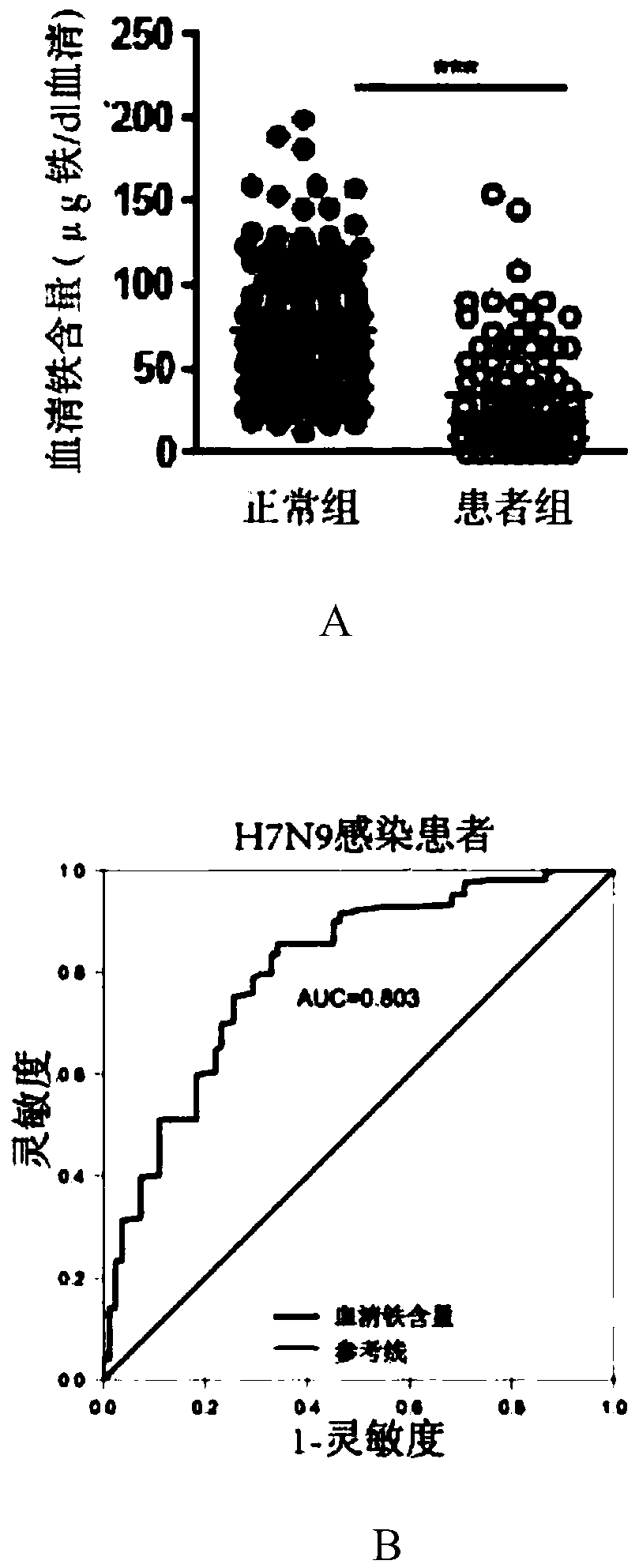
[0032] Experiment 1. Low serum iron content in patients infected with H7N9 avian influenza:
[0033] 1.1 Serum samples
[0034] Serum samples were selected from: 42 serum samples of H7N9 avian influenza patients and 83 normal human serum samples.
[0035] These serum samples were all from the First Affiliated Hospital of Zhejiang University School of Medicine. Among them, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com