A kind of preparation method of benzofuran compound
A technology of benzofuran and its compounds, which is applied in the field of benzofuran synthesis, can solve the problems of long routes, difficulty in obtaining, and many types of initial raw materials, and achieve the effect of simple raw material components and convenient process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
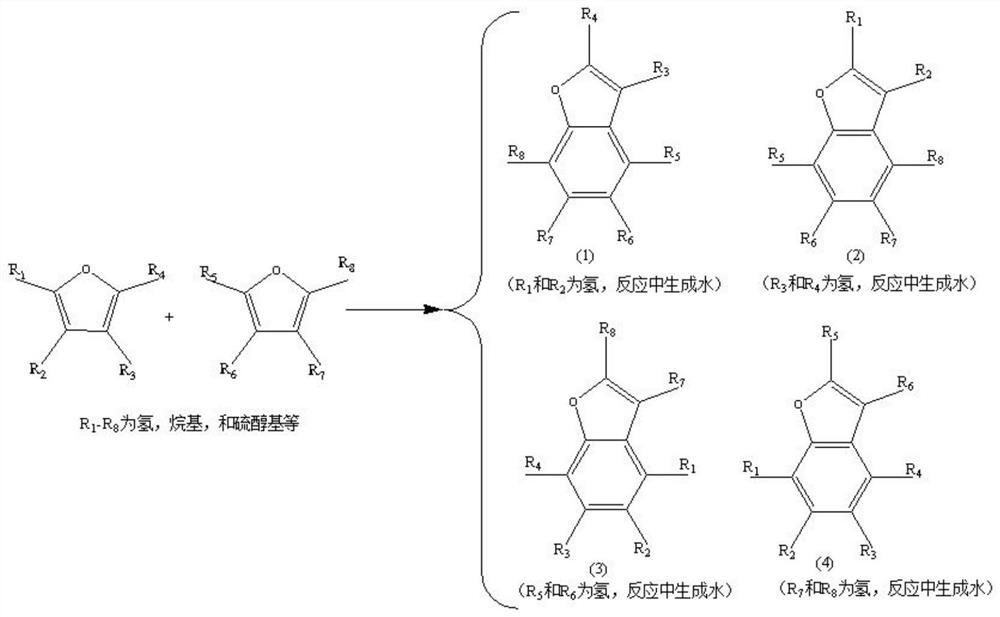
[0043]A method of synthesizing benzofuran in furan, as follows (reactive formula)figure 1 ):
[0044]The volume ratio of acetic acid with acetic acid and water was 3: 1 aqueous acetic acid solution, and 2 mL of the aqueous acetic acid solution was added to a 10 mL of the reaction tube, and 0.02 g of furan and 0.1 g of aluminum chloride catalyst were added to the reaction tube.
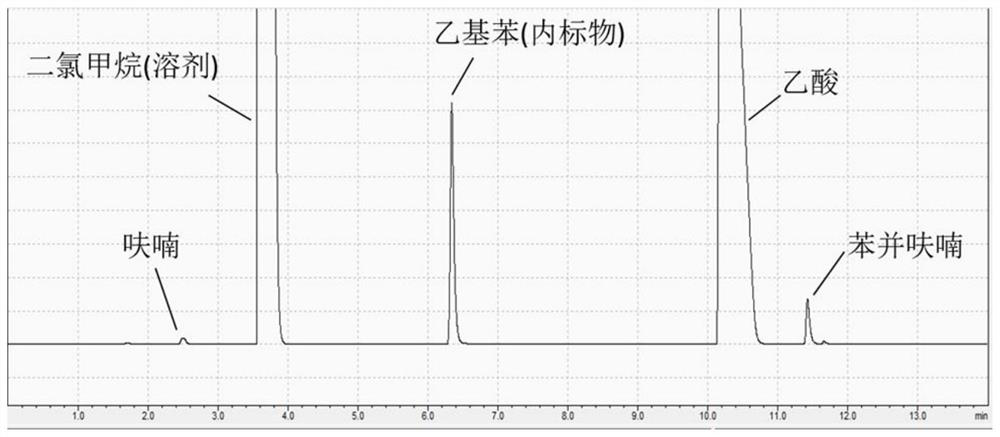
[0045]The reaction tube was reacted at 140 ° C for 1 hour, and benzofuran was obtained. After adding the reaction product to the inner label ethylbenzene, dissolved in dichloromethane, a quantitative analysis of furan and benzofuran in dichloromethane using GC-FID (seefigure 2 ). The furan conversion is 30.5%, and the yield of benzofuran is 25.8%, and the selectivity of benzofuran is as high as 99.1%. The solvent and benzofuran product were recovered by rectification, and the aqueous solution of solvent acetic acid was distilled under 100 ° C, and the benzofuran was further distilled off at 170 to 175 ° C.
Embodiment 2
[0047]A method of mixing benzofuran in furan, said method is as follows:
[0048]The volume ratio of acetic acid and water was formulated with water, and a aqueous acetic acid solution of 3: 1 was added to a 10 ml of the aqueous solution of acetic acid, 0.02 g of furan and 0.04 g of aluminum chloride removal of 3 ml of the reaction tube were added.
[0049]The reaction tube was reacted for 6 hours at 140 ° C, and benzofuran was obtained. After adding the reaction product to the inner label ethylbenzene, dissolved in dichloromethane and quantitative analysis of furan and benzofuran in dichloromethane using GC-FID. The furan conversion rate was 35.3%, and the yield of benzofuran was 29.8%, and the selectivity of benzofuran was as high as 97.3%. The solvent and benzofuran product were recovered by rectification.
Embodiment 3
[0051]A method of mixing benzofuran in furan, said method is as follows:
[0052]The volume ratio of acetic acid with acetic acid and water was 3: 1 aqueous acetic acid solution, and 2 mL of the aqueous acetic acid solution was added to a 10 mL of the reaction tube, and 0.02 g of furan and 0.1 g of aluminum chloride catalyst were added to the reaction tube.
[0053]The reaction tube was reacted at 155 ° C for 1 hour, and then benzofuran was obtained. The furan conversion rate was 36.7%, and the yield of benzofuran was 27.2%, and the selectivity of benzofuran was as high as 85.5%. The solvent and benzofuran product were recovered by rectification.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com