Butyphthalide derivative as well as preparation method and application thereof
A derivative, the technology of butylphthalide, applied in the field of butylphthalide derivatives and its preparation, can solve the problems of restricting the wide application of acute cerebral ischemia, the overall curative effect is not high, and achieve low drug prices, good antithrombotic activity, chemical The effect of the simple and easy synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
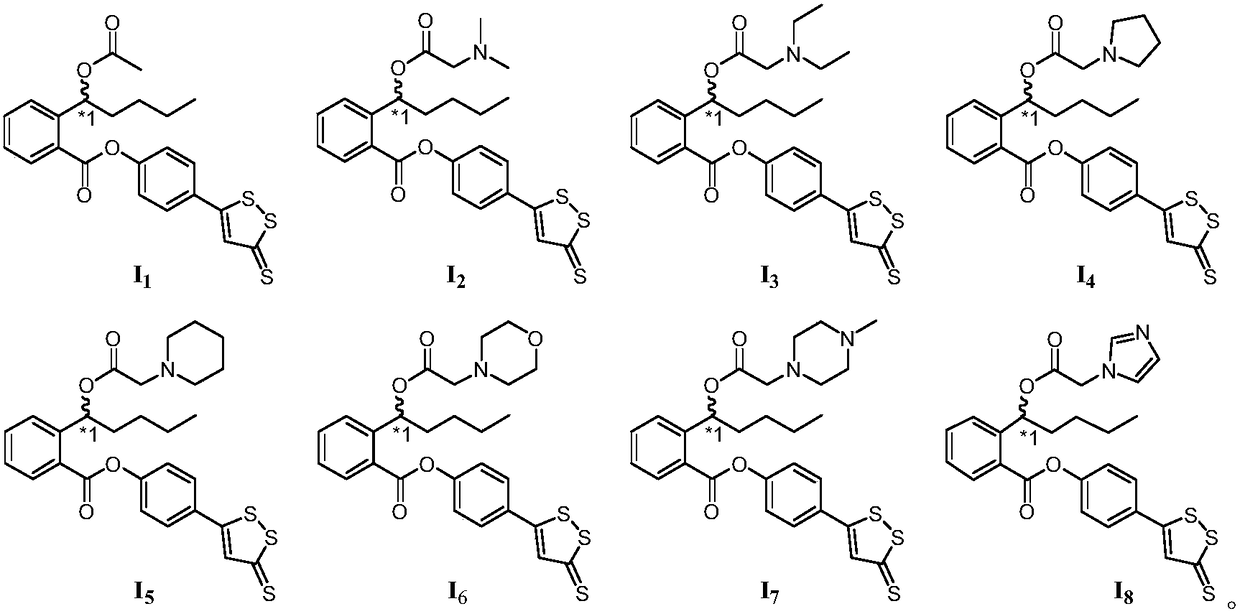
[0056] Example 1 Compound I 1 preparation of
[0057] Dissolve compound 3 (1.0mmol) in 20mL of anhydrous dichloromethane, add DCC (1.1mmol) and a catalytic amount of DMAP under stirring, halve the room temperature for 0.5h, then add compound 1 (1.0mmol) and react at room temperature for 6-8h . After the reaction was completed, it was filtered, concentrated under reduced pressure, and flash column chromatography (PE / EtOAc=15 / 1-5 / 1, v / v) obtained 366 mg of an orange solid product with a yield of 80%.
[0058] mp 94–96℃.MS(ESI):m / z 459.1[M+H] + .IR(cm -1 ,KBr):ν max 763, 1170, 1254, 1492, 1731. 1 H NMR (300Hz, CDCl 3 ):δ0.87(t,3H,CH 3 , J=6.8Hz), 1.26–1.50(m, 4H, 2×CH 2 ), 1.83–1.88 (m,2H,CH 2 ),2.08(s,3H,OOCCH 3 ),4.53(t,2H,OCH 2 ,J=6.0Hz),6.65(t,1H,CH,J=6.6Hz),7.40–7.44(m,3H,ArH),7.45(s,1H,C=CH),7.60–7.65(m,2H , ArH), 7.73–7.76 (m, 2H, ArH), 8.12 (d, 1H, ArH, J=7.8Hz). 13 C NMR (75Hz, CDCl 3 ): δ170.4, 164.7, 153.8, 144.7, 136.1, 133.4, 130.6, 130.3, 129.4, 129.4...
Embodiment 2
[0059] Example 2 Compound I 2 preparation of
[0060] Dissolve compound 5a (1.0mmol) in 20mL of anhydrous dichloromethane, add DCC (1.1mmol) and a catalytic amount of DMAP under stirring, halve the room temperature for 0.5h, then add compound 1 (1.0mmol) and react at room temperature for 6-8h . After the reaction was completed, it was filtered, concentrated under reduced pressure, and subjected to flash column chromatography (PE / EtOAc=15 / 1-5 / 1, v / v) to obtain 434 mg of an orange-yellow oily product with a yield of 84%.
[0061] MS(ESI):m / z 502.3[M+H] + .IR(cm -1 ,KBr):ν max 764, 1169, 1276, 1490, 1739. 1 HNMR (300Hz, CDCl 3 ):δ0.85(t,3H,CH 3 ,J=6.8Hz),1.26–1.45(m,4H,2×CH 2 ), 1.86–1.90 (m,2H,CH 2 ),2.33(s,6H,CH 3 NCH 3 ),3.20–3.21(m,2H,OCOCH 2 N),6.65(t,1H,CH,J=6.4Hz),7.40–7.45(m,3H,ArH),7.46(s,1H,C=CH),7.60–7.62(m,2H,ArH), 7.73–7.76(m,2H,ArH),8.12(d,1H,ArH,J=7.8Hz). 13 C NMR (75MHz, CDCl 3 ): δ170.1, 164.7, 153.8, 144.5, 136.1, 133.3, 130.7, 130.4, 129.4, 128....
Embodiment 3
[0062] Example 3 Compound I 3 preparation of
[0063] Dissolve compound 5b (1.0mmol) in 20mL of anhydrous dichloromethane, add DCC (1.1mmol) and a catalytic amount of DMAP under stirring, halve the room temperature for 0.5h, then add compound 1 (1.0mmol) and react at room temperature for 6-8h . After the reaction was completed, it was filtered, concentrated under reduced pressure, and subjected to flash column chromatography (PE / EtOAc=15 / 1-5 / 1, v / v) to obtain 434 mg of an orange-yellow oily product with a yield of 82%.
[0064] MS(ESI):m / z 530.2[M+H] + .IR(cm -1 ,KBr):ν max 764, 1169, 1400, 1491, 1738. 1 HNMR (300Hz, CDCl 3 ):δ0.85(t,3H,CH 3 ,J=6.8Hz),0.98(t,6H,2×NCH 2 C H 3 ,J=7.1Hz), 1.26–1.45(m, 4H, 2×CH 2 ), 1.86–1.96 (m,2H,CH 2 ),2.65(q,4H,2×NC H 2 CH 3,J=7.1Hz),3.32–3.47(m,2H,OCOCH 2 N),6.63(t,1H,CH,J=6.5Hz),7.40–7.43(m,3H,ArH),7.44(s,1H,C=CH),7.60–7.62(m,2H,ArH), 7.73–7.76(m,2H,ArH),8.12(d,1H,ArH,J=7.8Hz). 13 C NMR (75MHz, CDCl 3 ): δ170.1, 164.7, 15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com