A kind of method that solvent method prepares calcium bicarbonate powder
A technology of calcium bicarbonate and solvent method, applied in the direction of calcium carbonate/strontium/barium, etc., can solve problems such as difficult synthesis of calcium bicarbonate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Dissolve calcium chloride in ethanol to a concentration of about 40mmol / L; then add triethylamine to an equal volume of ethanol to a concentration of about 80mmol / L. After mixing evenly, pass in carbon dioxide gas under stirring, filter with a sand core funnel after white turbidity is formed, and wait for the solvent to volatilize at dry room temperature to obtain high-purity calcium bicarbonate powder with a purity of 100%.
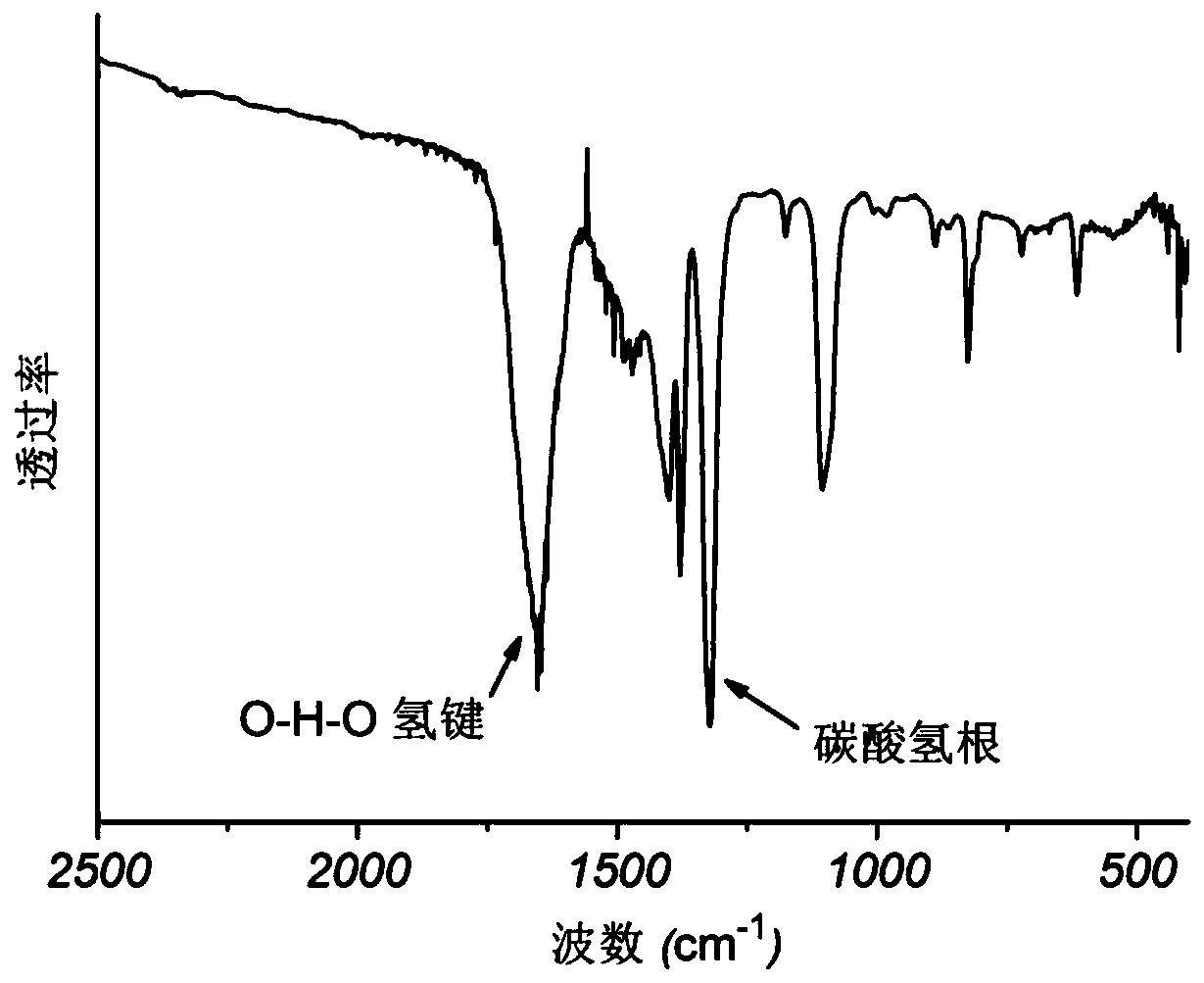
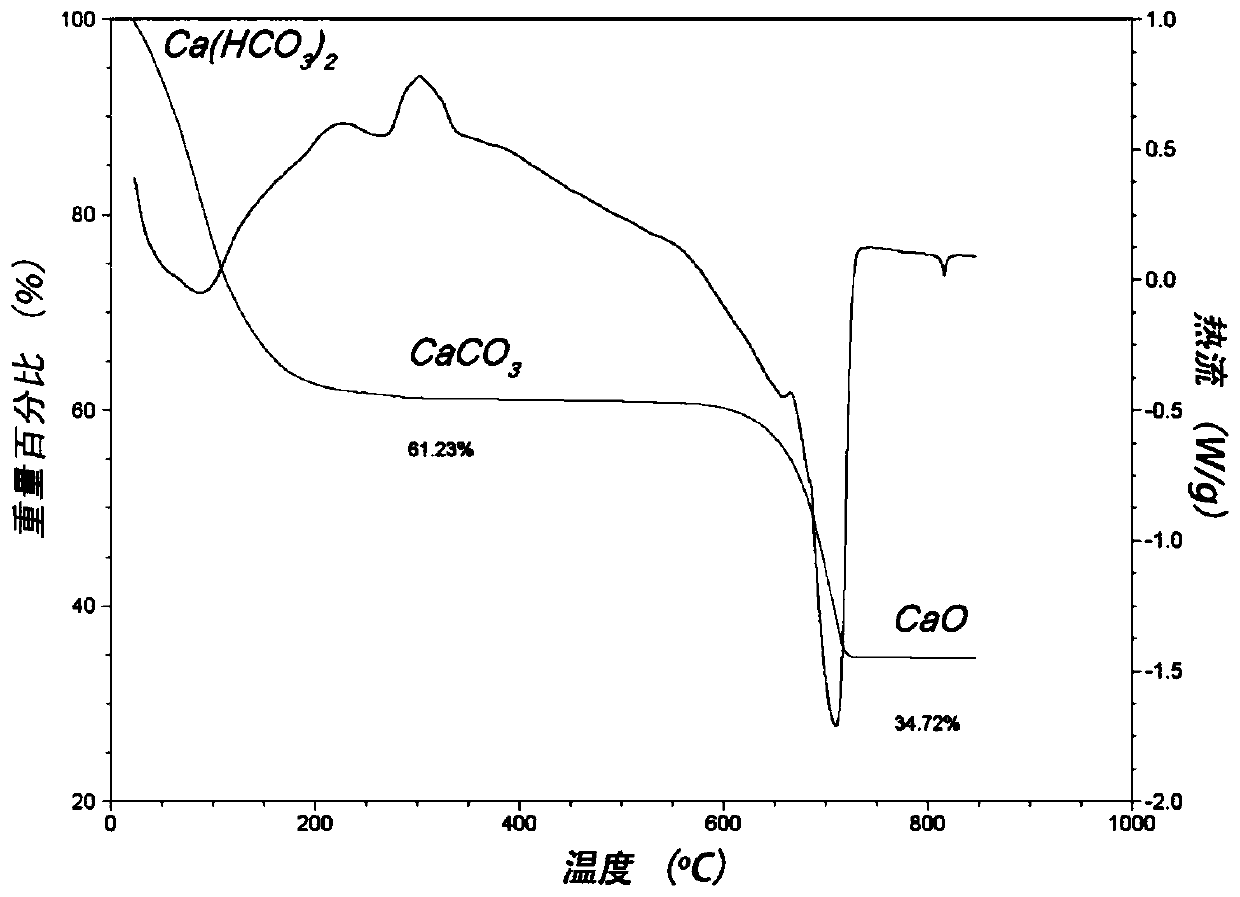
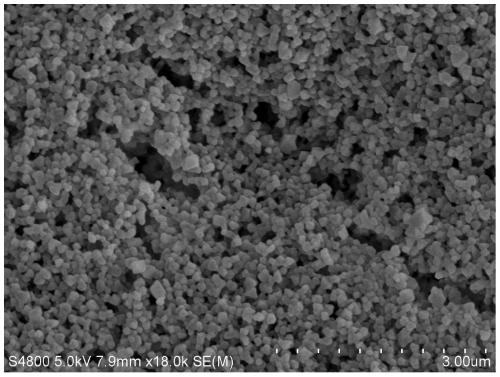
[0039] Such as Figure 1-4 As shown, the calcium bicarbonate powder prepared in Example 1 has been respectively characterized by infrared spectroscopy, thermogravimetric analysis, scanning electron microscopy and X-ray diffraction. from figure 1 It can be seen that the product has characteristic peaks of bicarbonate and O-H-O hydrogen bonds, which are completely different from the spectral peaks of calcium carbonate; from figure 2 As can be seen from the weight loss curve in, the purity of product is 100%; From image 3 It can be seen that the...
Embodiment 2
[0041] Dissolve calcium chloride dihydrate in acetone so that the concentration reaches about 15mmol / L, and then add triethylamine to an equal volume of acetone until the concentration is about 37.5mmol / L. After mixing evenly, pass a sufficient amount of carbon dioxide gas into the solution containing triethylamine. Afterwards, the two solutions were mixed under stirring, centrifuged after white turbidity was formed, and the supernatant was removed. After the solvent is volatilized at room temperature, high-purity calcium bicarbonate powder can be obtained.
[0042] The calcium bicarbonate powder prepared in this embodiment has also been characterized by infrared spectroscopy, thermogravimetric analysis, scanning electron microscopy and X-ray diffraction, which proves that the product is calcium bicarbonate nanopowder with a purity of 96%. The yield was 100% by gravimetric analysis.
Embodiment 3
[0044] Dissolve calcium nitrate in methanol to a concentration of about 20mmol / L, then add triethanolamine to an equal volume of methanol to a concentration of about 10mmol / L. After mixing evenly, inject carbon dioxide gas under stirring, centrifuge after forming white turbidity, and dry under reduced pressure at room temperature. After the solvent evaporates, high-purity calcium bicarbonate powder can be obtained.
[0045] The calcium bicarbonate powder prepared in this embodiment has also been characterized by infrared spectroscopy, thermogravimetric analysis, scanning electron microscopy and X-ray diffraction, which proves that the product is calcium bicarbonate nanopowder with a purity of 96%. The yield was 22% by gravimetric analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com