Preparation method and use of rivaroxaban intermediate
A technology of rivaroxaban and intermediates, applied in the preparation of rivaroxaban intermediate I, the field of preparing high-purity rivaroxaban, can solve the problems of long reaction time, large solvent amount, waste of energy and the like, and achieves The effect of easy industrial production, short reaction time and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
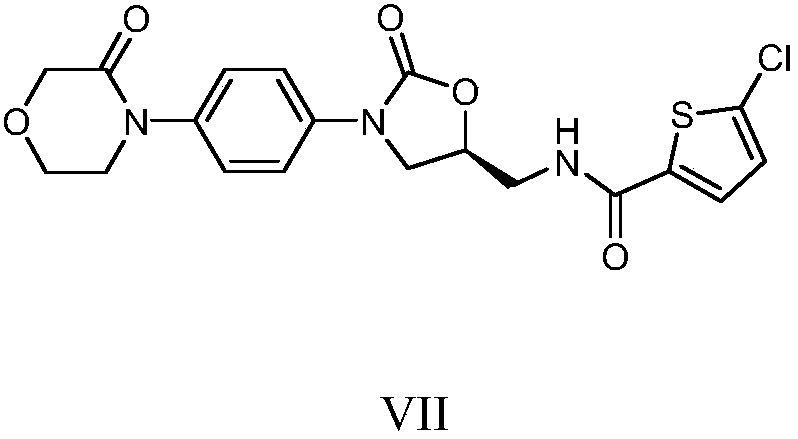
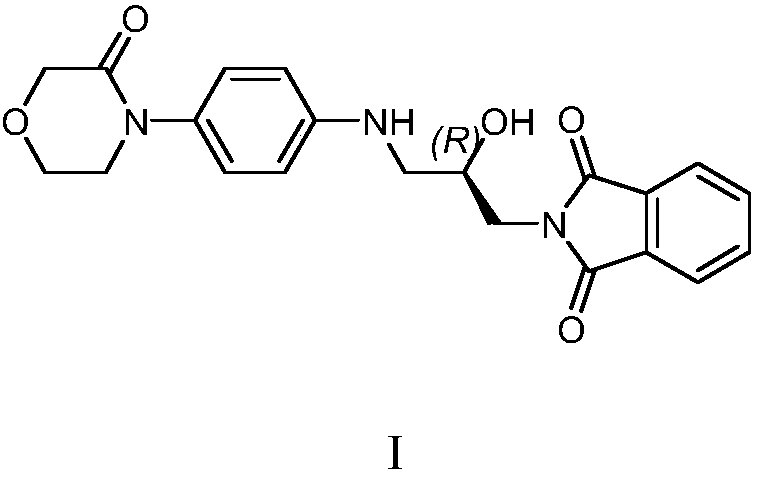
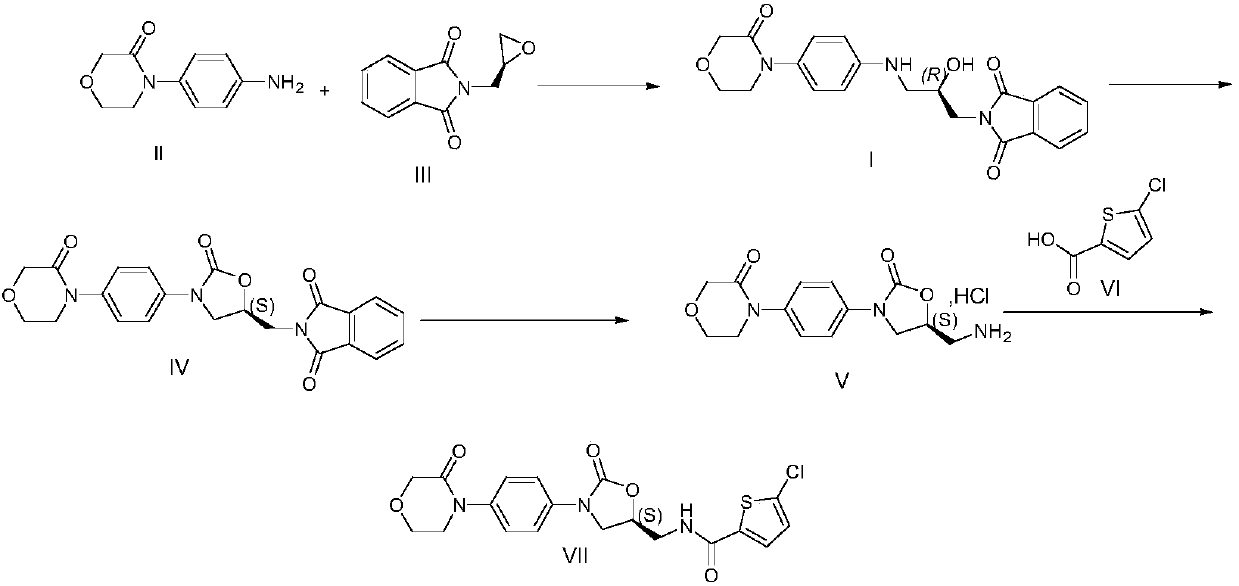
[0020] Example 1 Preparation of Rivaroxaban Intermediate I
[0021] 20kg 4-(4-aminophenyl) morpholin-3-one, 27.5kg (S)-(+)-N-(2,3-ethoxypropyl) phthalimide, 600kg Add purified water into a 1000L glass-lined reaction tank, stir and heat up to 75-85°C for 5 hours, cool down to room temperature, centrifuge, and dry the filter cake to obtain 39.1kg of rivaroxaban intermediate I with a yield of 95.0% and a purity of 96.90%.
[0022] MS (ESI, m / z): 396.17 (M+1)
[0023] 1 H NMR (300MHz, DMSO): δ=7.82~7.89(m,4H); 7.01~7.04(d,2H); 6.60~6.63(d,2H); 5.65~5.69(m,1H); 5.17~5.18( d,1H); 4.14(s,2H); 4.00~4.02(m,1H); 3.91~3.95(m,2H); 3.59~3.66(m,4H); 3.15~3.17(m,1H); 3.02~ 3.04(m,1H).
Embodiment 2
[0024] Example 2 Preparation of Rivaroxaban Intermediate I
[0025] 35.0g of 4-(4-aminophenyl)morpholin-3-one, 37.0g of (S)-(+)-N-(2,3-ethoxypropyl)phthalimide, Add 875mL of purified water into a 2000mL three-necked flask, stir and heat up to 80-90°C for 3 hours, cool down to room temperature, filter with suction, and dry the filter cake to obtain 68.1g of rivaroxaban intermediate I with a yield of 94.6% and a purity of 96.42%.
Embodiment 3
[0026] Example 3 Preparation of Rivaroxaban Intermediate I
[0027] 2.5kg of 4-(4-aminophenyl)morpholin-3-one, 3.42kg of (S)-(+)-N-(2,3-ethoxypropyl)phthalimide, Add 100L of purified water into a 200L glass-lined reaction tank, stir and heat up to 70-80°C for 4 hours, cool down to room temperature, centrifuge, and dry the filter cake to obtain rivaroxaban intermediate I 4.86kg, yield 94.5%, purity 96.59% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com