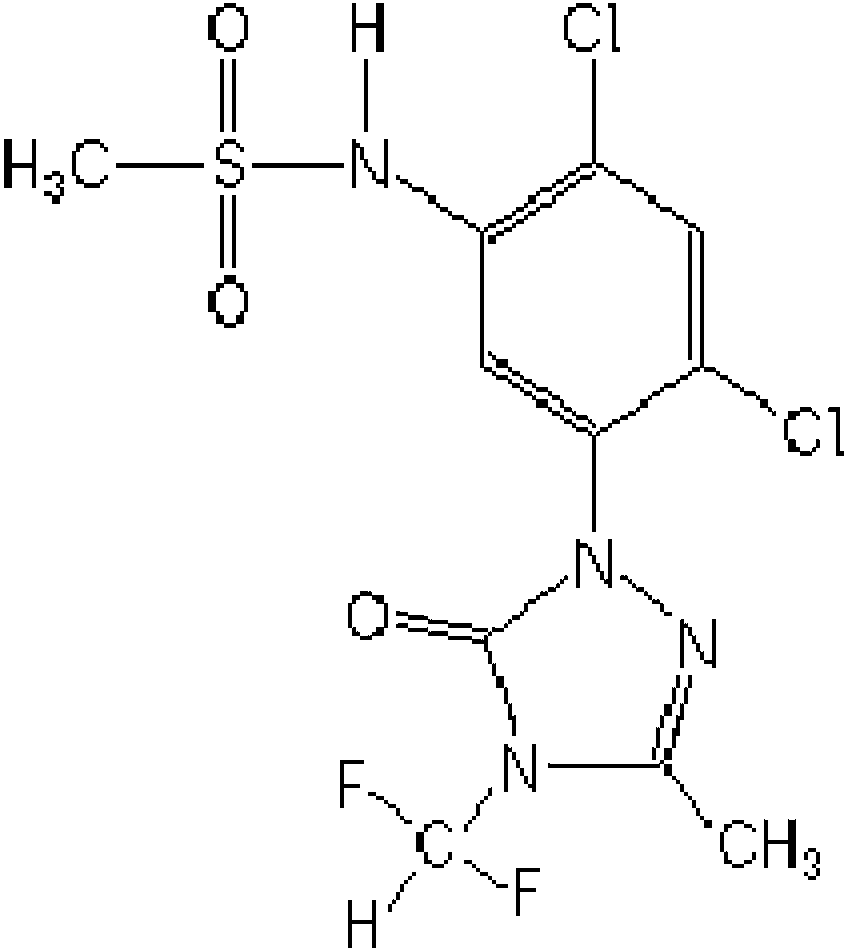
A process for preparing a novel formulation of sulfentrazone and use of the same
A technology of sulfentralam and refined metolachlor, which is applied in the field of preparation and application of new sulfentrachlor preparations, and can solve problems such as the adverse effects of sulfentralam function and the decrease of sulfentrachlor content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 - Samples were prepared as follows:
[0048] Dissolve sulfentrachlor and purified metolachlor in acetone.
[0049] Table 1
[0050]
Embodiment 19
[0052] An emulsifiable concentrate with the following composition was prepared:
[0053]
[0054]
[0055] Examples 1 to 18 were tested to determine their photolytic stability using the following procedure:
[0056] Add 20 mL of the solution to the quartz tube. The tube is continuously irradiated with UV light from a UV lamp. Aliquots (100 [mu]L) of this solution were removed from the tube at 2 hours and 8 hours. The concentration of sulfentrazone in each aliquot was determined by HPLC.
[0057] The results are summarized in Table 2 below.
[0058] Table 2
[0059]
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com