Methods for administration and methods for treating cardiovascular diseases with resiniferatoxin
A resin toxin and cardiovascular technology, applied in cardiovascular system diseases, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve problems affecting nerves, etc., and achieve the effect of easy application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
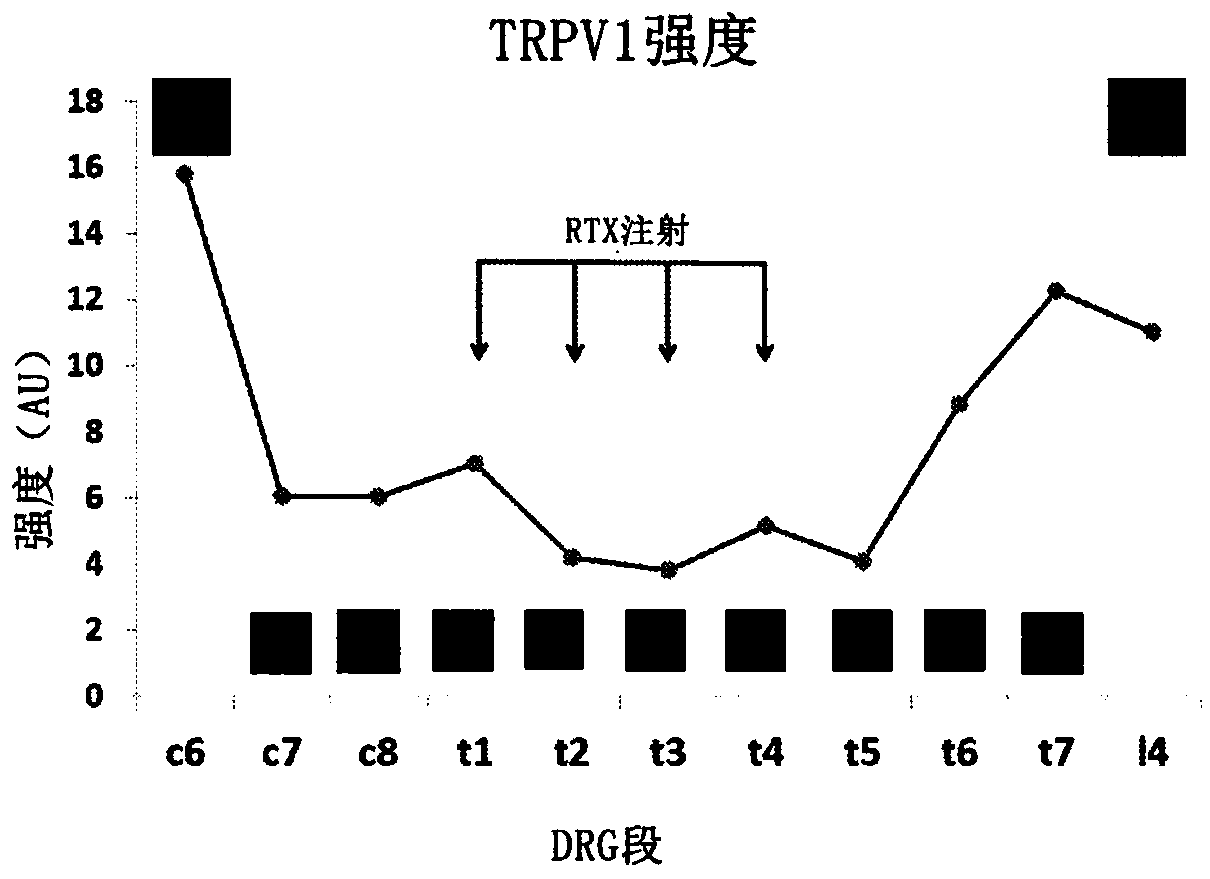
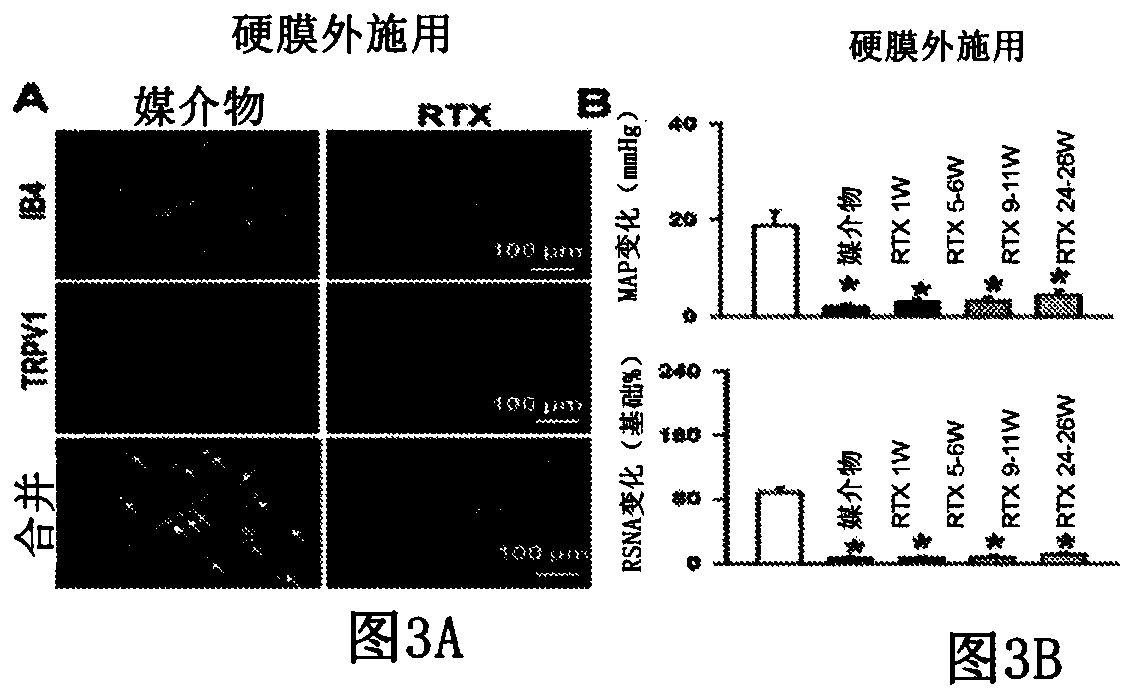
[0070] Figures 3A to 4 B shows the experimental results of a rat study according to one embodiment of the present application. Figure 3A Shown are experimental data from a rat model showing isolectin B4 (IB4) and TRPV1 responses in rat populations that did not receive epidural RTX injections and that received epidural RTX injections. Figure 3B Experimental data from a rat model showing mean arterial pressure (MAP) and renal sympathetic nerve activity measured over a 26-week period in the RTX-naïve (vehicle) and epidural RTX-treated groups (RSNA). Figure 3C Experimental data from a rat model are shown showing MAP and RSNA measured over a 26 week period for the RTX-naïve (vehicle) and epicardial RTX-treated populations.
[0071] Figure 3A It was shown that isolectin B4 (IB4) and TRPV1 expression was reduced in DRG neurons of various sizes after RTX treatment compared to vehicle-only treatment which showed visually significantly increased expression. Figure 3B Responses...
Embodiment 2
[0074] Figure 4 Experimental results of a rat study according to one embodiment of the present application are shown. Figure 4Images showing the dorsal horn of the T2 spinal cord stained for TRPV1 and substance P (SP), comparing subjects receiving RTX injections to control subjects. Epidural application of RTX ablated nearly all SP-containing C-fiber afferents (peptidergic) and most isolectin B4 (IB4)-positive C-fiber afferents projecting to the dorsal horn of the thoracic spinal cord at the T1 to T4 DRG level ( non-peptidergic). Figure 4 showed decreased expression of TRPV1 protein and disruption of IB4-containing cell bodies, indicating ablation of small-diameter neurons. Ablation of neurons in the dorsal horn of the spinal cord expressing SP by RTX. IB4 is an indicator of small diameter afferent nerves and SP is an indicator of neuroinflammation. In each case, the reduced expression was evident from the reduced signal in the images of RTX-treated subjects compared to...
Embodiment 3
[0076] Figure 5 Experimental results of a rat study according to one embodiment of the present application are shown. Figure 5 Experimental data showing cardiac function in each of four Sprague-Dawley rat populations: sham-operated rats administered vehicle only (column A), sham-operated rats administered RTX epidurally (column B), induced chronic Rats with heart failure (CHF) administered vehicle only (column C) and rats with induced chronic heart failure (CHF) and epidurally administered RTX (column D), (n=9-16 per group). Experimental data include: body weight, heart weight, ratio of heart weight to body weight (HW / BW), ratio of wet lung weight to body weight (WLW / BW), left ventricular end systolic pressure (LVESP), left ventricular end diastolic pressure (LVEDP ), the maximum value of the first derivative of left ventricular pressure (dp / dt max ), the minimum value of the first derivative of left ventricular pressure (dp / dt min ) and infarct size. Statistically signi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com