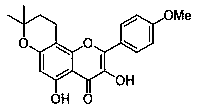
Method for preparing dewatered icaritin through total-synthesis
A dehydrated icariin, total synthesis technology, applied in the production of bulk chemicals, organic chemistry and other directions, can solve the problems of many side compounds, expensive catalysts, complicated processes, etc., and achieves simple operation, low reaction equipment requirements, and reasonable methods. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
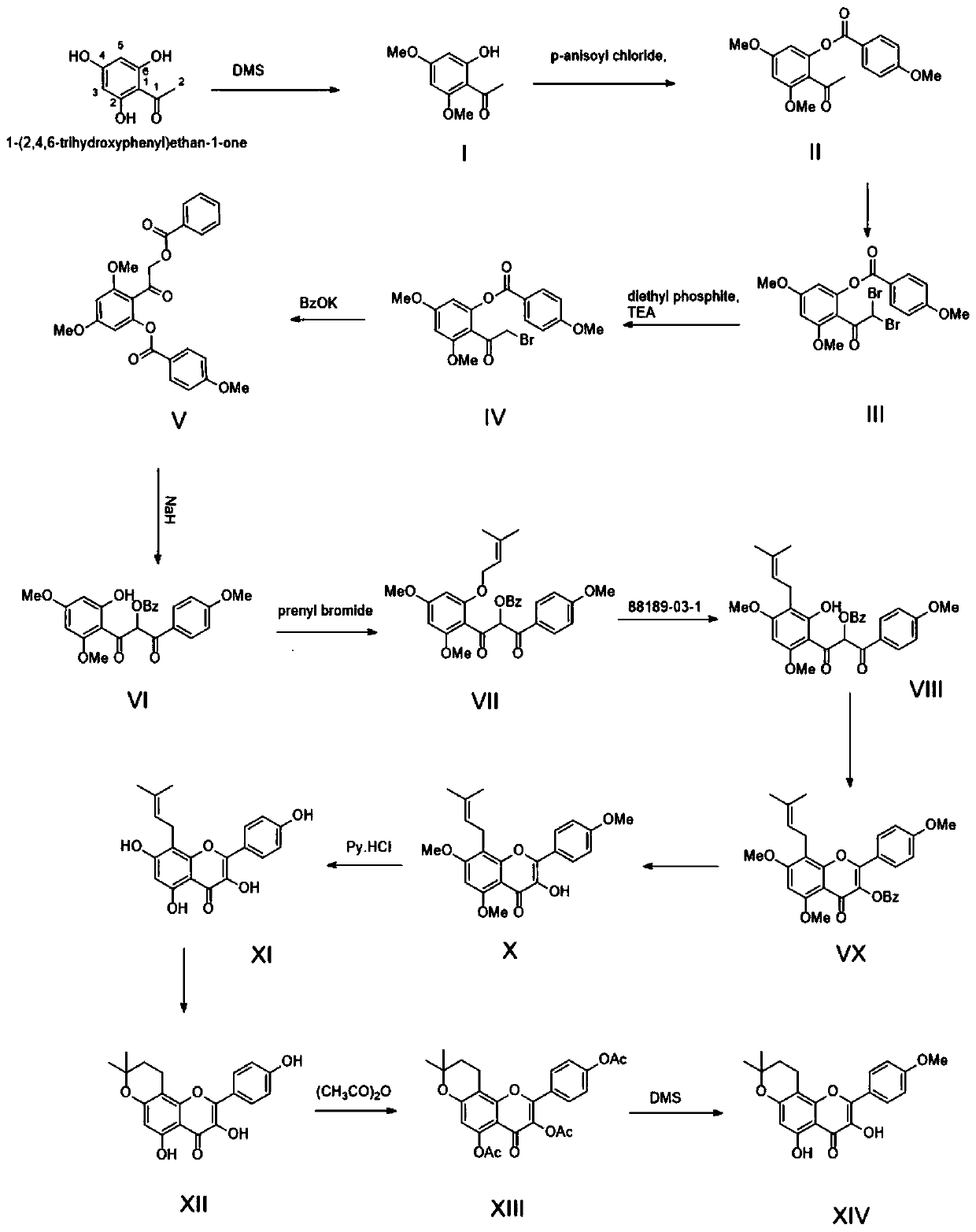
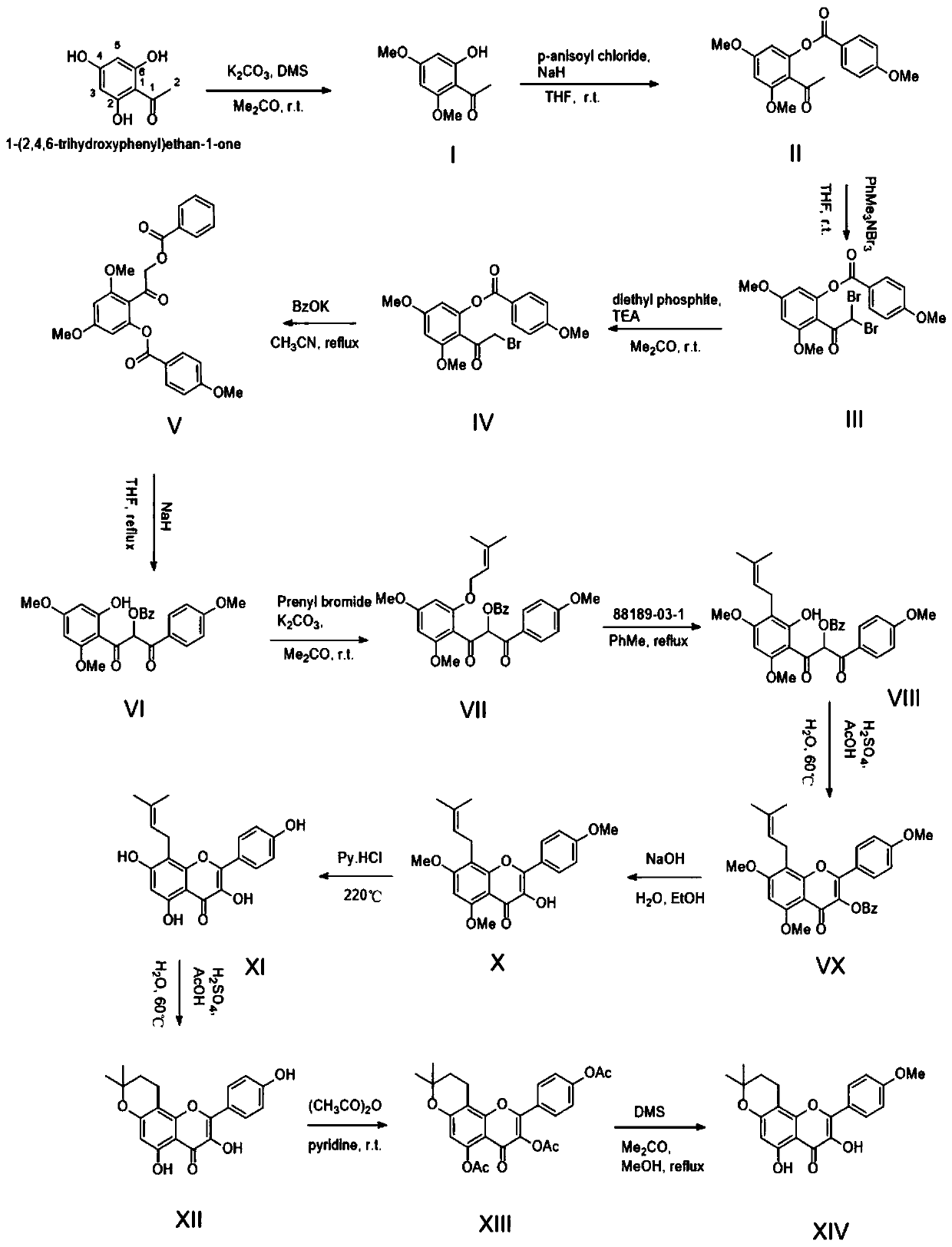
[0076] A kind of method of fully synthetically preparing dehydrated icariin, such as figure 2 Shown synthetic route, comprises the following steps:
[0077] 1. hydroxyl protection
[0078] Dissolve trihydroxyacetophenone (16.8g, 0.1mol) in acetone (500ml), add anhydrous potassium carbonate (13.8g, 0.1mol), heat up to 50°C and reflux; take dimethyl sulfate (25.2g, 0.2 mol), add one-third to the reaction solution every 3 hours, put it under reflux at 50°C for 9 hours, filter the reaction solution, evaporate the filtrate to dryness, and obtain a yellow solid, which is washed with water three times and then dried at low temperature to obtain off-white Solid product I (14.8g), yield 76%
[0079] 2. Introduce the B ring
[0080] Dissolve the product I (19.6g, 0.1mol) in anhydrous tetrahydrofuran (500ml), add 1 times the amount of NaH (2.4g, 0.1mol) after cooling down to 0°C, add 1.2 times the amount of p-methoxybenzene after stirring for 5min Formyl chloride (20.5g, 0.12mol), ...
Embodiment 2
[0106] A kind of method of fully synthetically preparing dehydrated icariin, such as figure 2 Shown synthetic route, comprises the following steps:
[0107] 1. hydroxyl protection
[0108] Dissolve trihydroxyacetophenone (16.8g, 0.1mol) in 500ml of acetone, add anhydrous potassium carbonate (41.4g, 0.3mol), heat up to 60°C and reflux; take dimethyl sulfate (25.2g, 0.2mol) , add one-third to the reaction solution every 3 hours, put it under reflux at 50°C for 9 hours, then filter the reaction solution, evaporate the filtrate to dryness to obtain a yellow solid, wash it with water three times and dry it at low temperature to obtain an off-white solid product I (15.3 g), yield 79%.
[0109] 2. Introduce the B ring
[0110] Dissolve the product I (19.6g, 0.1mol) in anhydrous tetrahydrofuran (500ml), add 2 times the amount of NaH (4.8g, 0.2mol) after cooling down to 0°C, add 1.2 times the amount of p-methoxybenzene after stirring for 5min Formyl chloride (20.5g, 0.12mol), the...
Embodiment 3
[0136] A kind of method of fully synthetically preparing dehydrated icariin, such as figure 2 Shown synthetic route, comprises the following steps:
[0137] 1. hydroxyl protection
[0138] Dissolve trihydroxyacetophenone (16.8g, 0.1mol) in 500ml of acetone, add anhydrous potassium carbonate (27.6g, 0.2mol), heat up to 56°C and reflux; take dimethyl sulfate (25.2g, 0.2mol) , add one-third to the reaction solution every 3 hours, put it under reflux at 56°C for 9 hours, filter the reaction solution, evaporate the filtrate to dryness, and obtain a yellow solid, wash it with water three times, and dry it at low temperature to obtain an off-white solid product I (16.3g), 83% yield.
[0139] 2. Introduce the B ring
[0140] Dissolve the product I (19.6g, 0.1mol) in anhydrous tetrahydrofuran (500ml), add 1.5 times the amount of NaH (3.6g, 0.15mol) after cooling down to 0°C, add 1.2 times the amount of p-methoxybenzene after stirring for 5min Formyl chloride (20.5g, 0.12mol), the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com