A kind of preparation method of 1,1,1,2,3-pentachloropropane
The technology of pentachloropropane and tetrachloropropane is applied in the field of preparation of 1,1,1,2,3-pentachloropropane, and can solve the problems of complex reaction and purification system, coking and blackening of reaction solution, corrosion of production system, etc. , to achieve the effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] In this example, 1,1,1,2,3-pentachloropropane is prepared by batch reaction preparation method, and the reaction process is as follows:
[0055] Response one:
[0056] Response two:
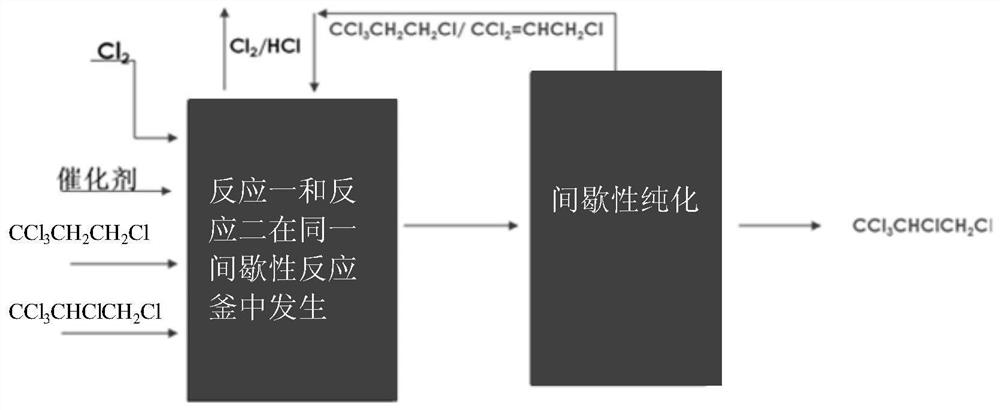
[0057] Such as figure 1 Shown, reaction one and reaction two described in the present invention take place successively in the same intermittent reactor, and two-step reaction is carried out in the intermittent reactor with jacket of same 20L, and intermittent reactor is equipped with mechanical stirring , A condenser is installed above the reactor, and the tail gas pipe is connected to the top of the condenser, and the acid tail gas is emptied after being absorbed by liquid alkali with a concentration of 15%.
[0058] The first step reaction: dehydrochlorination of 1,1,1,3-tetrachloropropane to generate 1,1,3-trichloropropene.
[0059] Put 16.8kg of 1,1,1,3-tetrachloropropane, 4.5kg of 1,1,1,2,3-pentachloropropane, 0.021kg of anhydrous ferric chloride into the reactor in turn, star...
Embodiment 2
[0064] In this example, 1,1,1,2,3-pentachloropropane is prepared by batch reaction preparation method, and the reaction process is as follows:
[0065] Response one:
[0066] Response two:
[0067] Such as figure 1 As shown, the two-step reaction is carried out in the same 20L jacketed reactor with mechanical stirring. A condenser is installed on the reactor, and the tail gas pipe is connected to the top of the condenser. The acid tail gas is absorbed by 15% liquid alkali and then emptied. .
[0068] The first step reaction: dehydrochlorination of 1,1,1,3-tetrachloropropane to generate 1,1,3-trichloropropene.
[0069] 11.0kg of 1,1,1,3-tetrachloropropane, 11.0kg of 1,1,1,2,3-pentachloropropane, 0.033kg of anhydrous ferric chloride were put into the reactor in turn, and the stirring was started, and the jacket was passed through Heating with hot water, passing -20°C frozen brine through the condenser, and starting timing when the temperature of the reaction solution ris...
Embodiment 3
[0074] In this example, 1,1,1,2,3-pentachloropropane is prepared by batch reaction preparation method, and the reaction process is as follows:
[0075] Response one:
[0076] Response two:
[0077] A 500mL jacketed flask is equipped with a mechanical stirrer. A condenser is installed on the top of the reactor. The top of the condenser is connected to a tail gas pipe. The acid tail gas is absorbed by 15% liquid alkali and then emptied.
[0078]The first step reaction: dehydrochlorination of 1,1,1,3-tetrachloropropane to generate 1,1,3-trichloropropene. Put 100g of 1,1,1,3-tetrachloropropane, 400g of 1,1,1,2,3-pentachloropropane, 0.05g of anhydrous ferric chloride into the reactor in turn, start stirring, and heat the jacket with hot water , the condenser is fed with -15°C frozen brine, the temperature of the reaction solution is raised to 70°C and the timing is started, and the second batch of ferric chloride 0.18g is added after 2h, and the temperature is continued at 70...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com