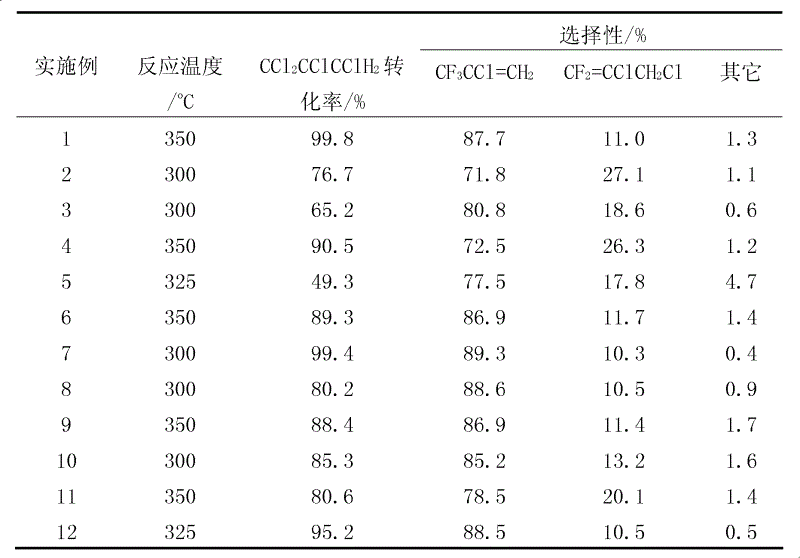
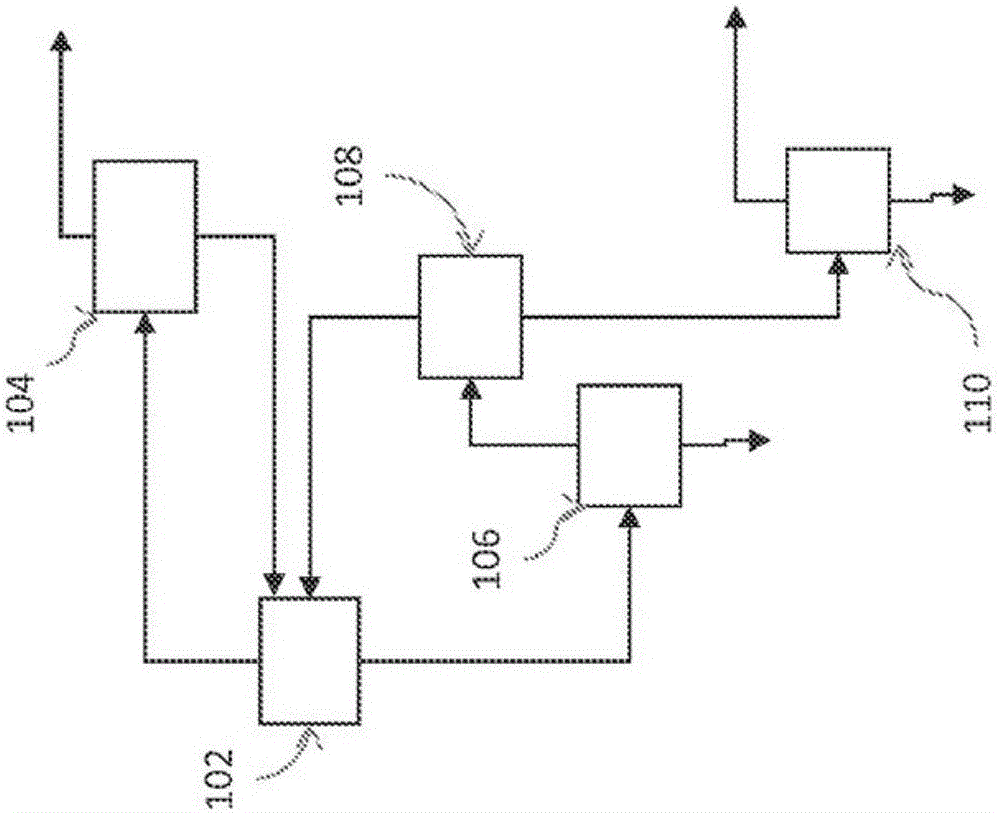
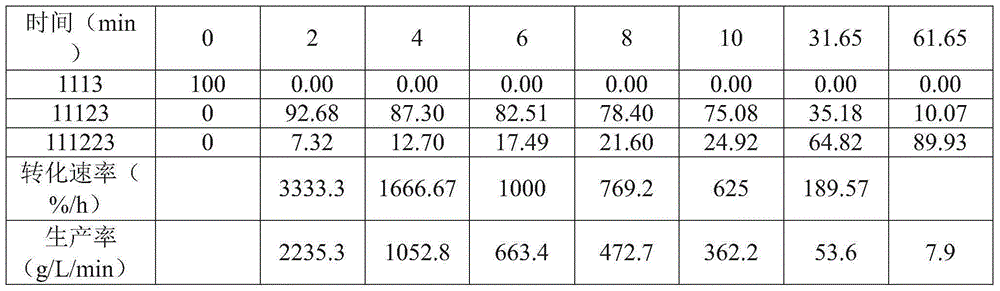
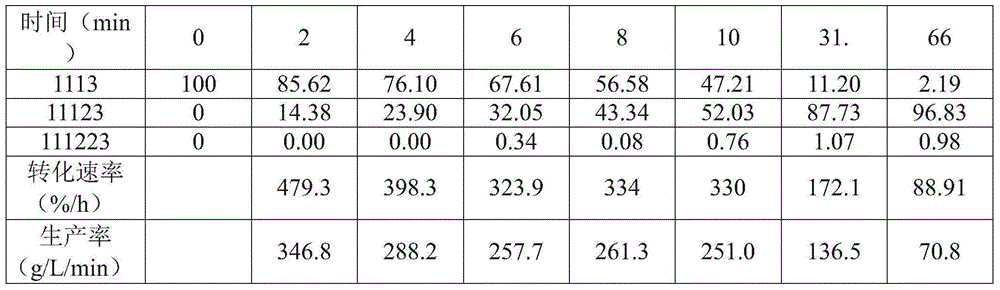
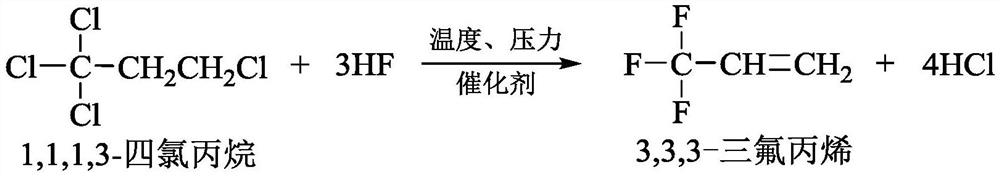
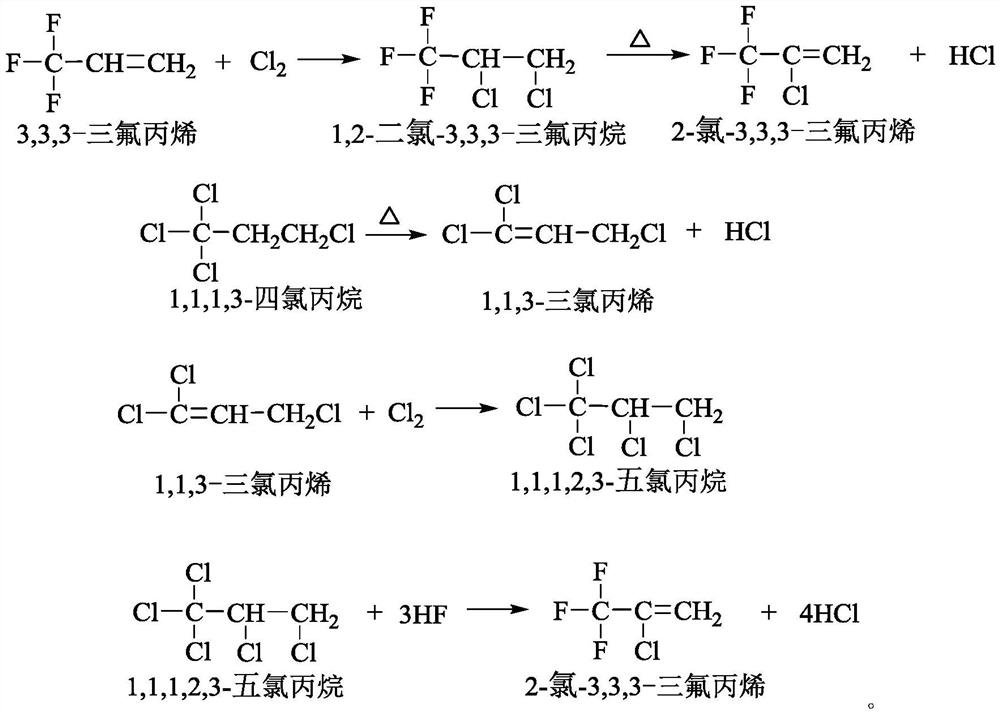
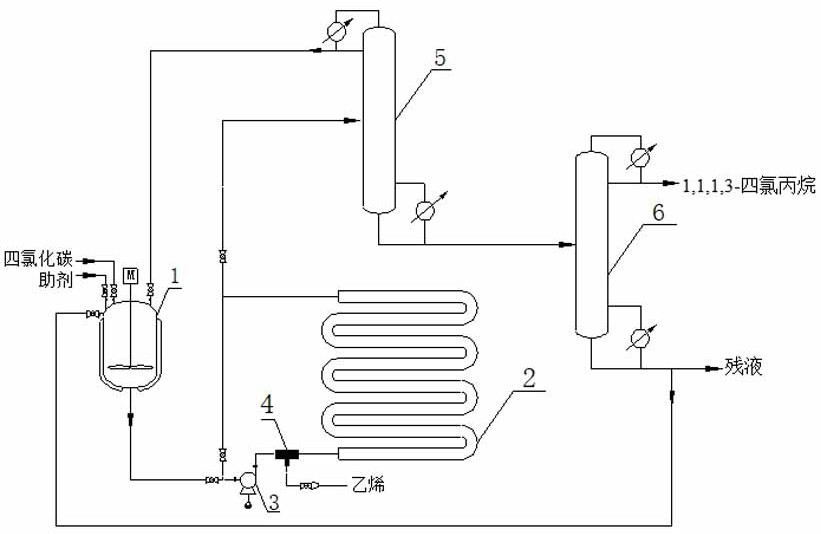
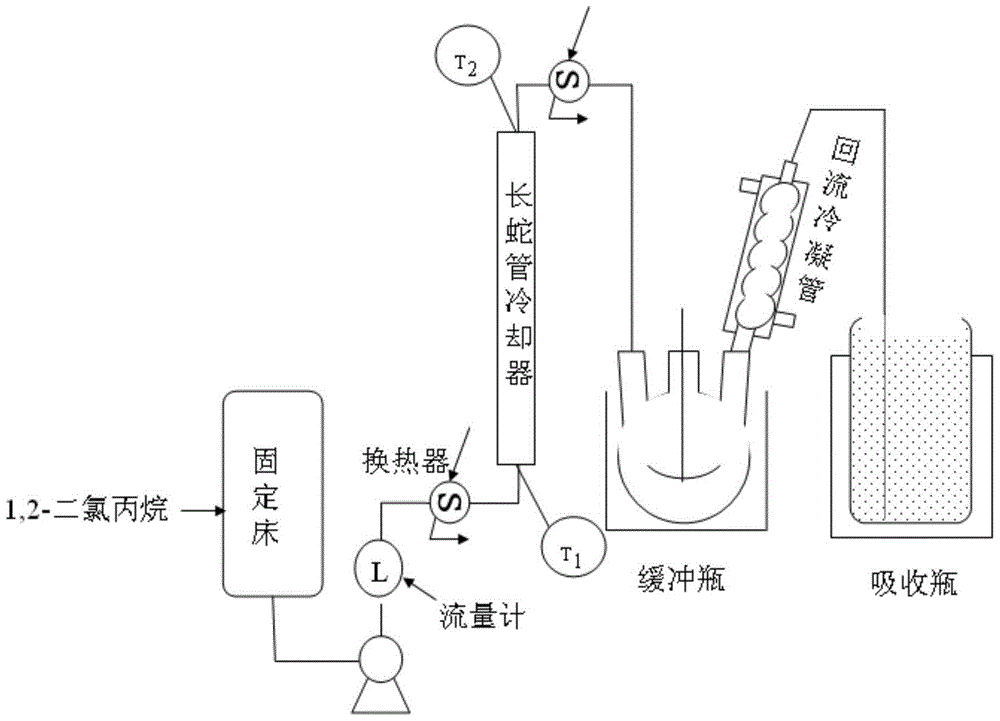
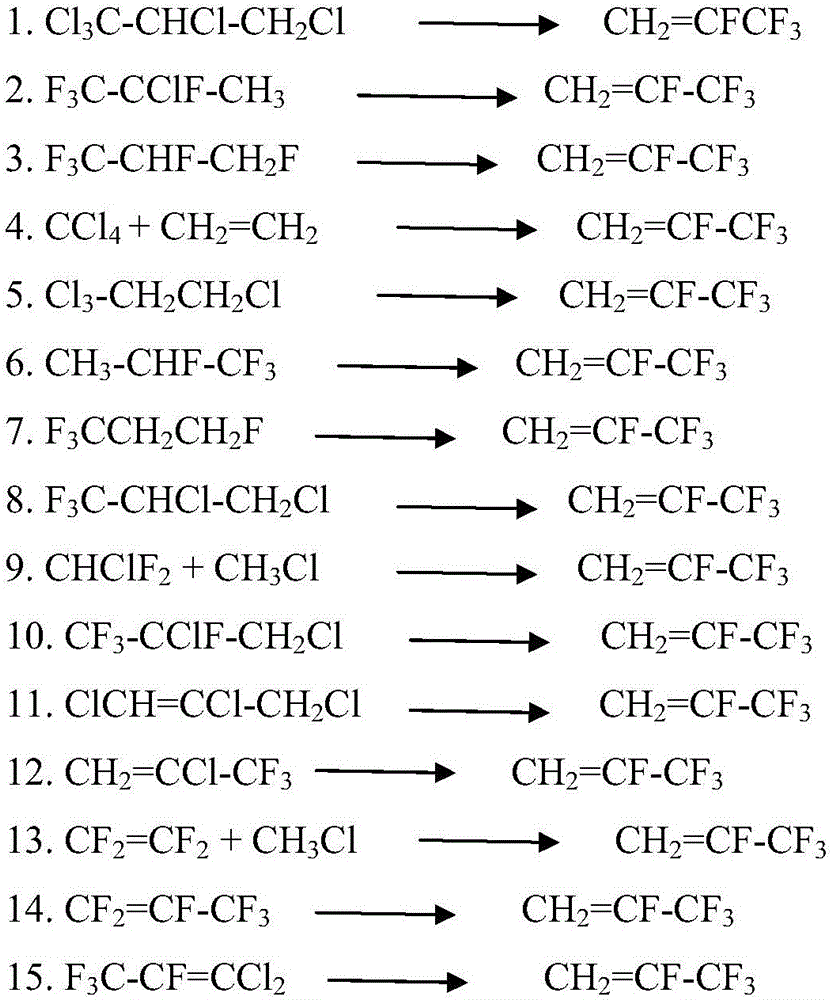
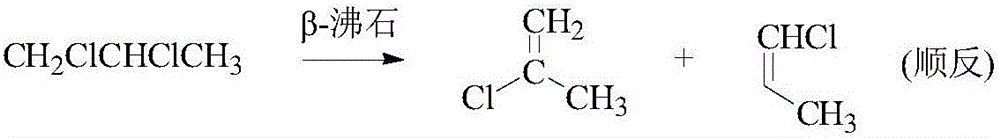
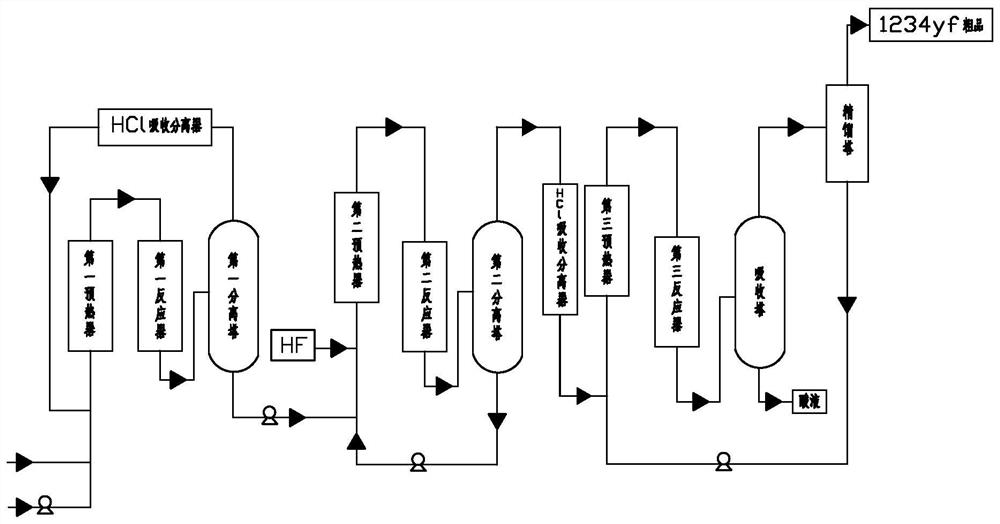
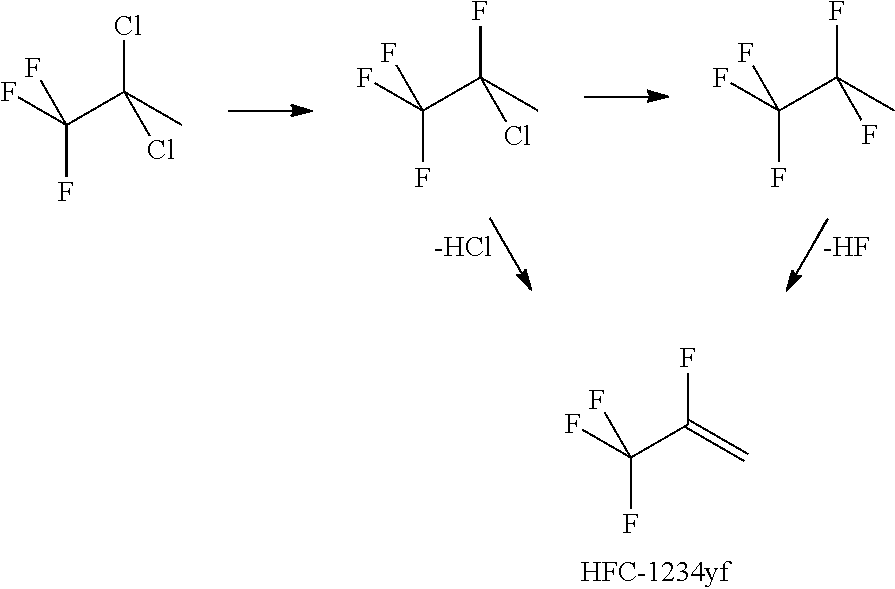
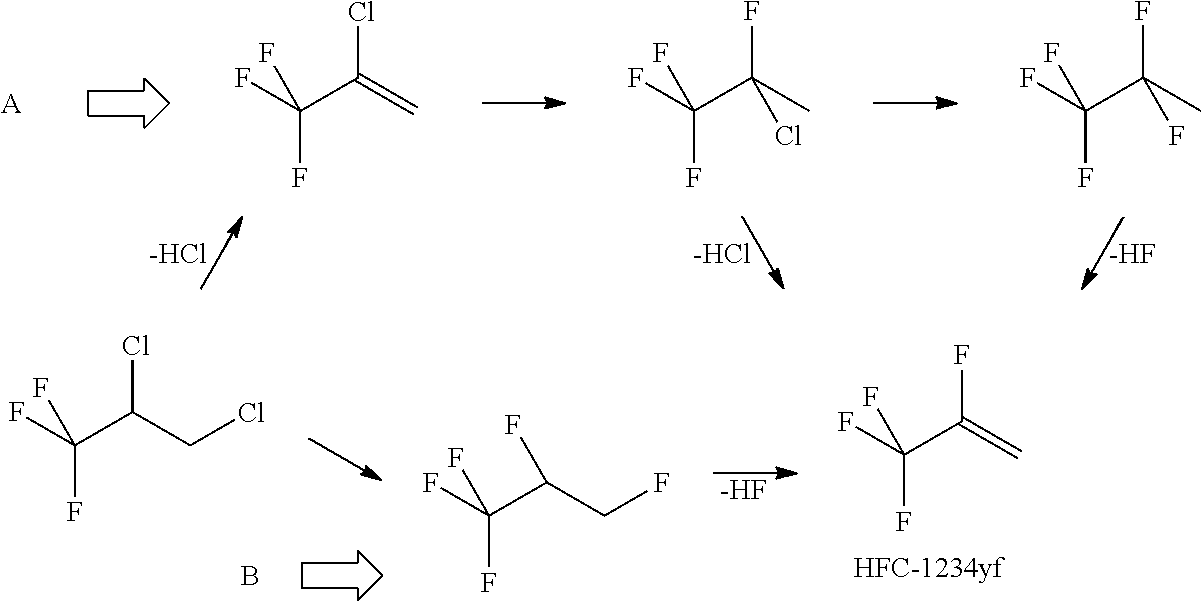
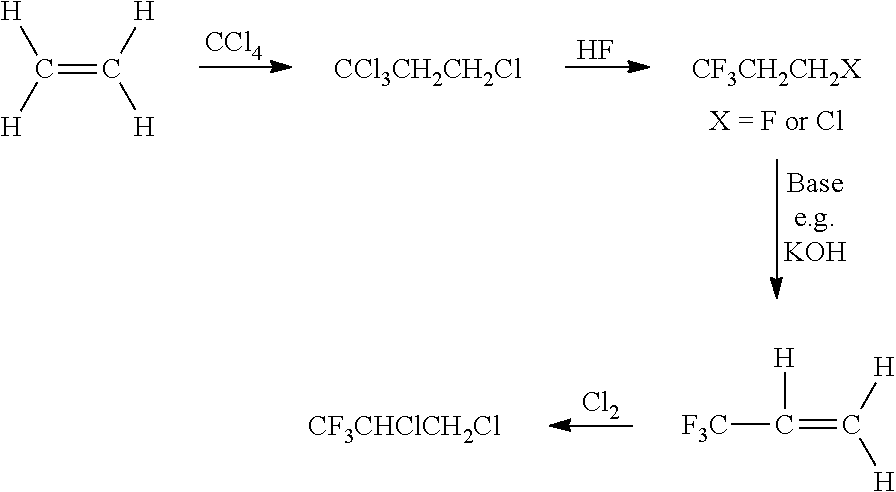
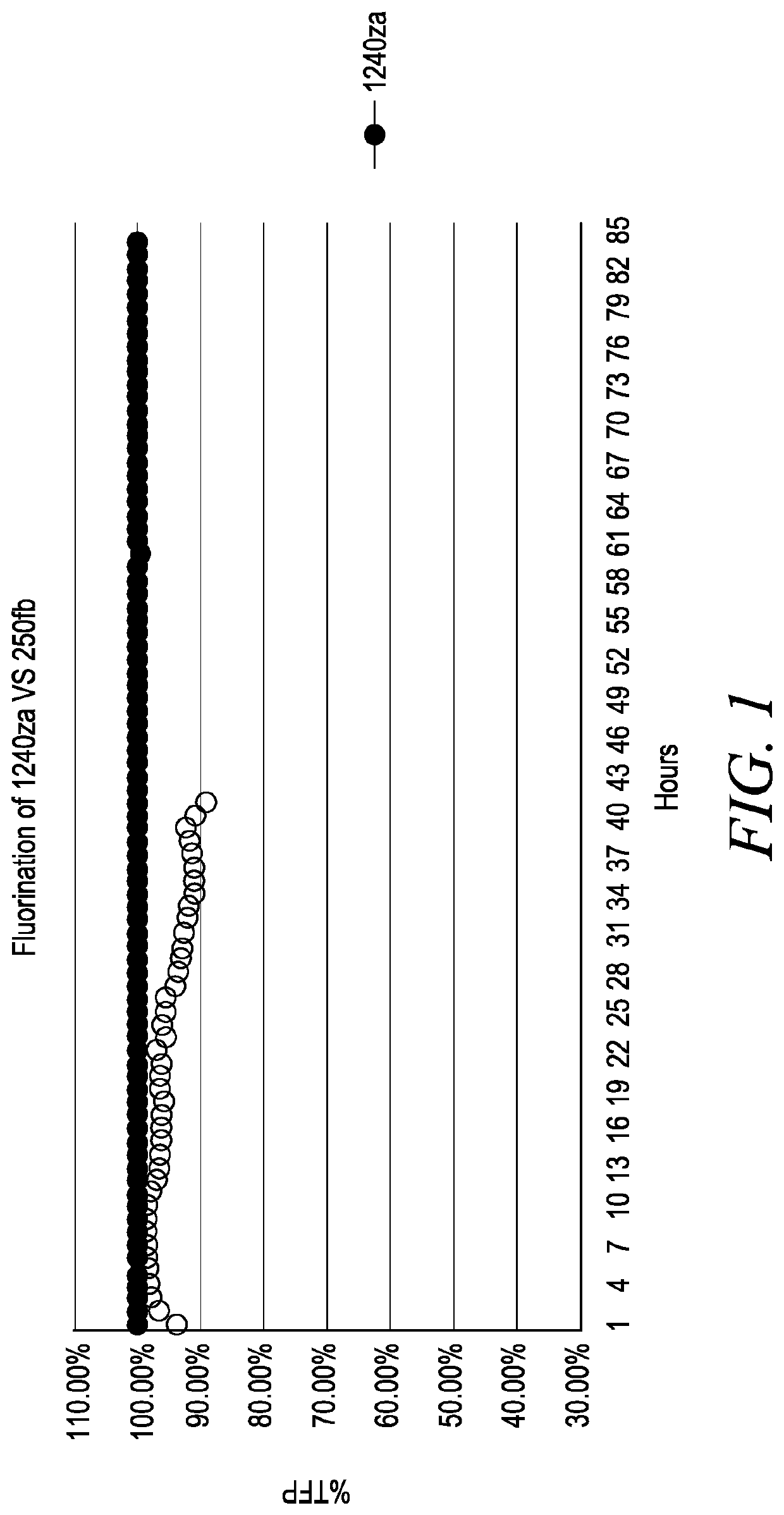
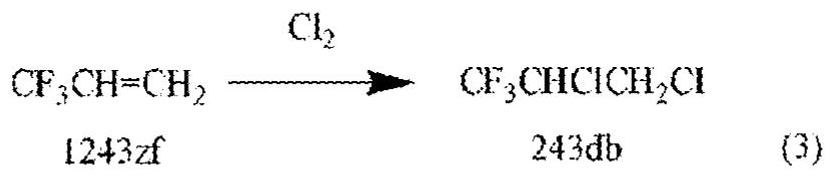
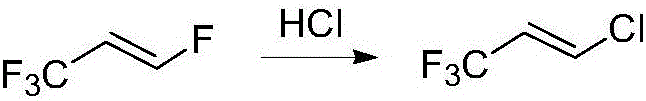
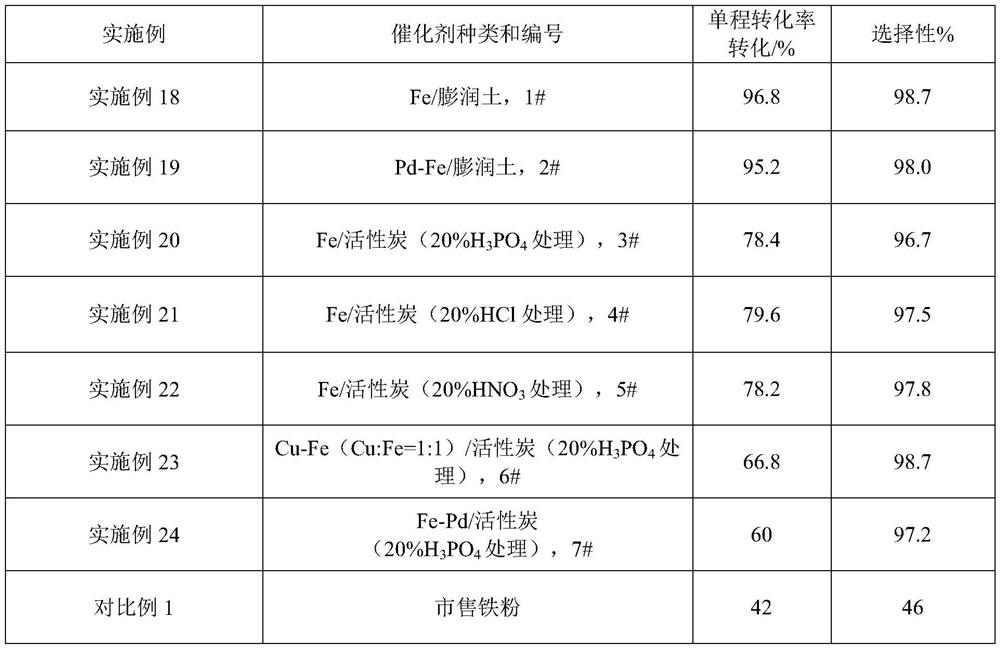
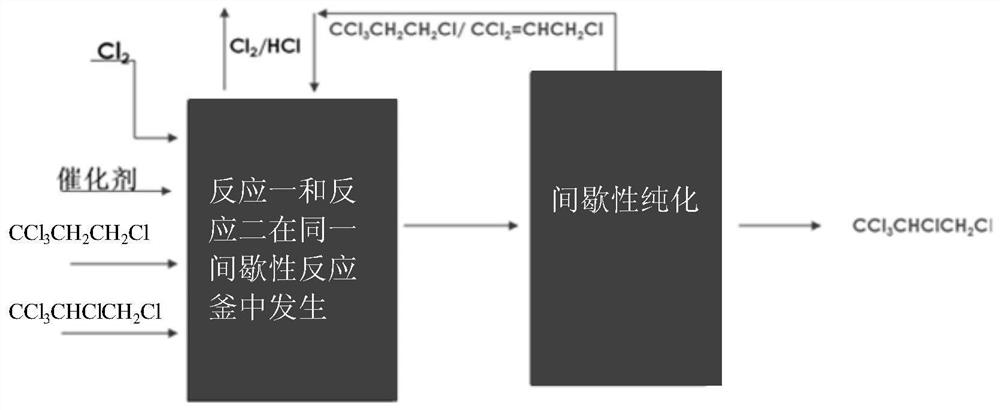
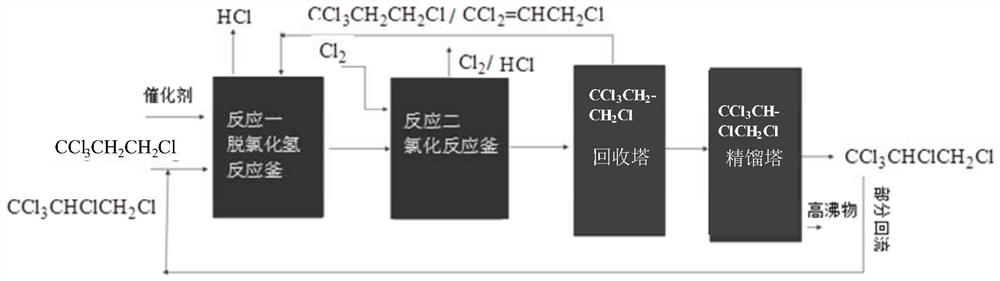
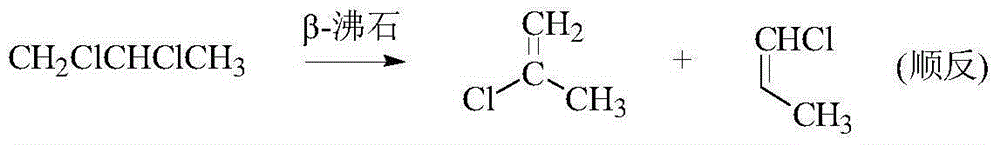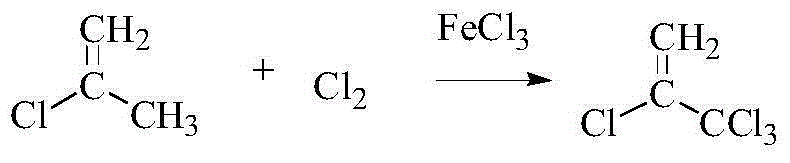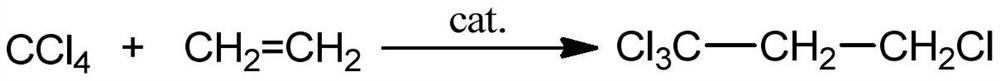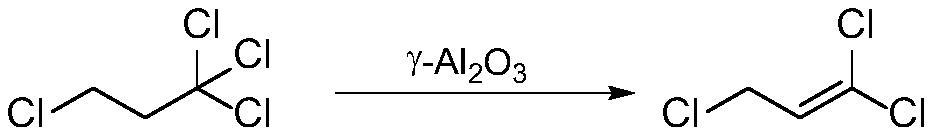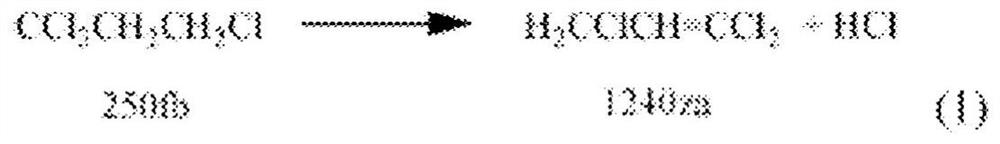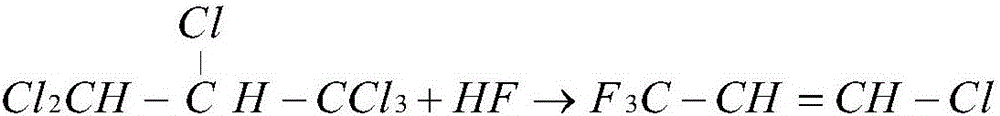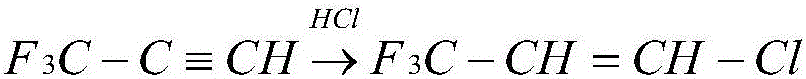Patents
Literature
31 results about "Tetrachloropropylene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
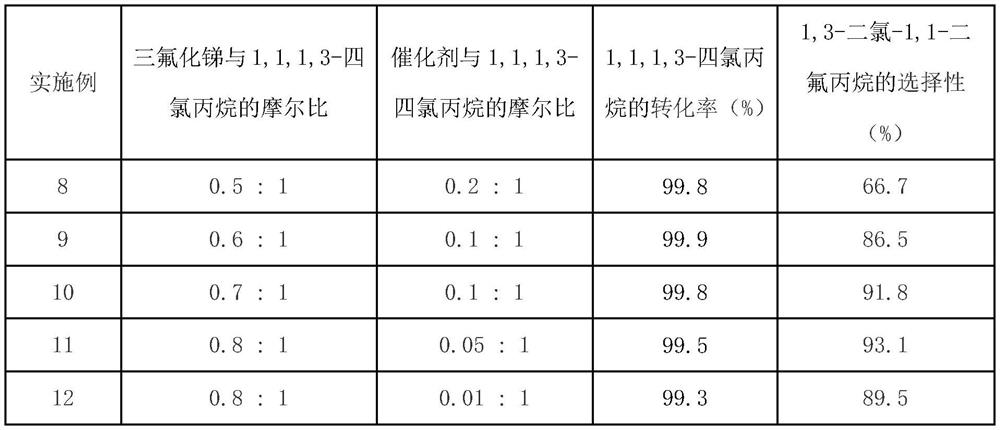
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Process
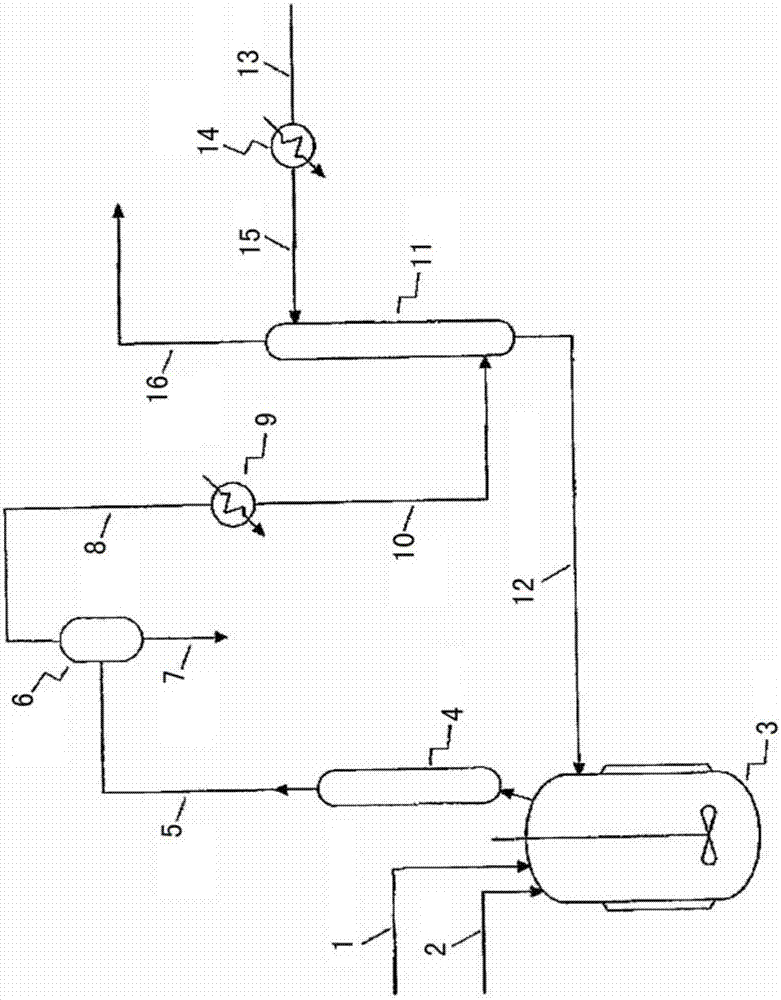
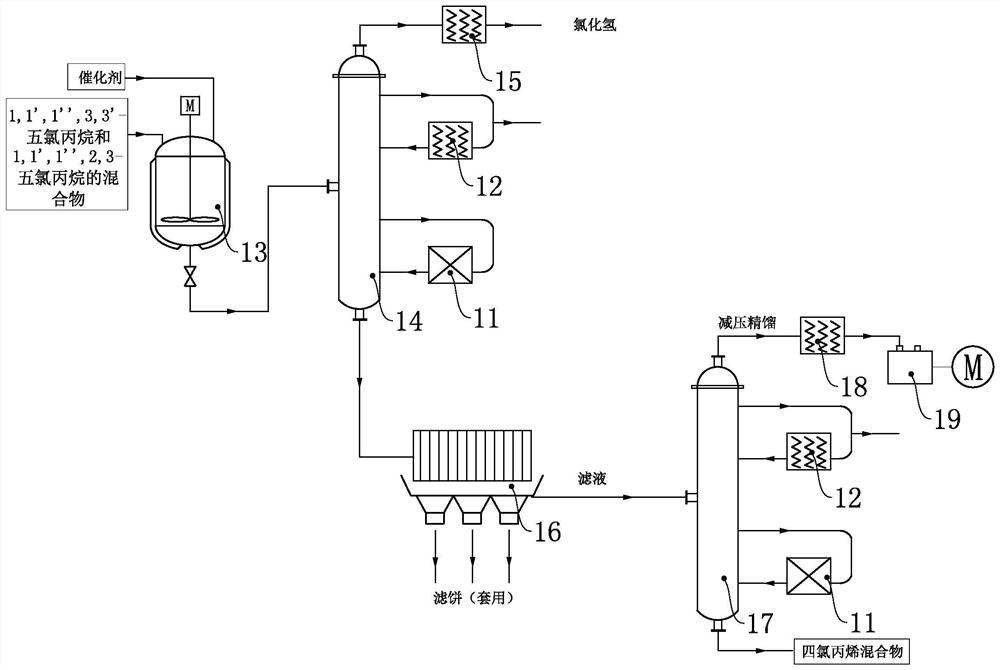
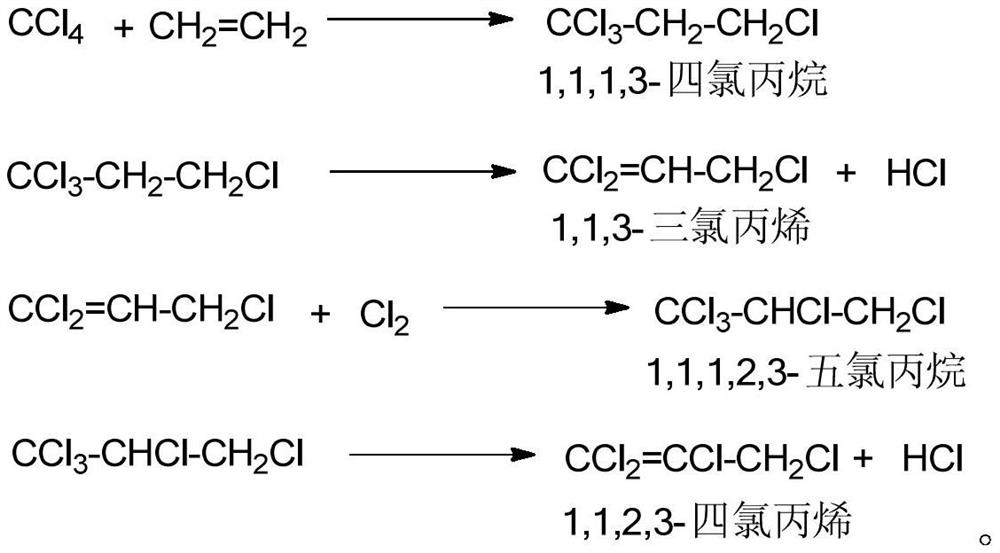
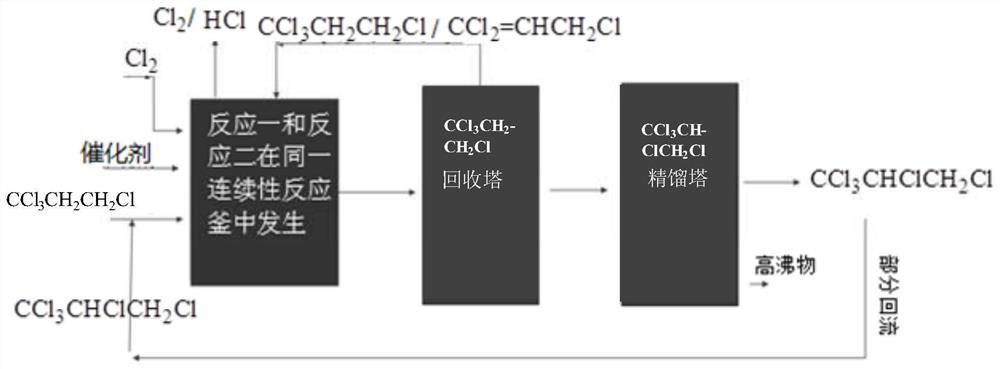
InactiveCN107001190ARealize continuous productionRealize comprehensive utilizationPreparation by hydrogen halide split-offPreparation by halogen replacementAlkyl transferReaction zone
Disclosed is a process for preparing a highly pure 1,1,1,2,3-pentachloropropane product. The method comprises 1-a) providing a reaction mixture comprising ethylene, carbon tetrachloride and a catalyst in a principal alkylation zone to produce 1,1,1,3-tetrachloropropane in the reaction mixture; 1-b) treating the reaction mixture obtained in step 1-a) to obtain a 1,1,1,3-tetrachloropropane feedstock; 2-a) contacting the 1,1,1,3-tetrachloropropane feedstock with a catalyst in a dehydrochlorination zone to produce a reaction mixture comprising 1,1,1,3-tetrachloropropane and 1,1,3-trichloropropene; 2-b) treating the reaction mixture obtained in step 2-a) to obtain a 1,1,3-trichloropropene feedstock; 3-a) contacting the 1,1,3-trichloropropene feedstock with chlorine in a reaction zone to produce a reaction mixture containing 1,1,1,2,3-pentachloropropane and 1,1,3-trichloropropene, the reaction zone being different from the dehydrochlorination zone; and 3-b) treating the reaction mixture obtained in step 3-a) to obtain the highly pure 1,1,1,2,3-pentachloropropane product.
Owner:SPOLEK PRO CHEMICKOU A HUTNI VYROBU
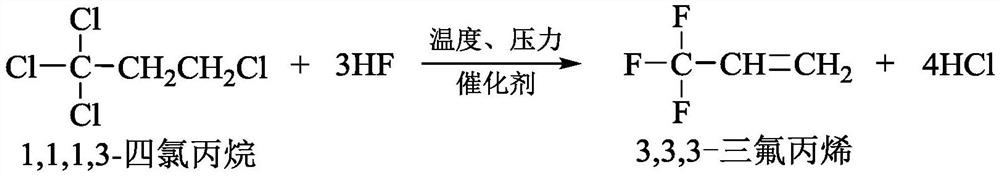
Preparation method for 3,3,3-trifluoropropene
ActiveCN103044191ALow costLow reaction temperaturePreparation by hydrogen halide split-offPtru catalystReaction temperature
The invention discloses a preparation method for 3,3,3-trifluoropropene, and the preparation method comprises the following steps of: (1) taking carbon tetrachloride and ethylene as a raw material and organic peroxides as a catalyst, and reacting to prepare 1,1,1,3-tetrachloropropane, wherein the molar ratio of carbon tetrachloride and ethylene is (1:1)-(30:1), the reaction pressure is 0.3-1.5MPa, the reaction temperature is 50-150DEG C, and the reaction time is 2-5 hours; (2) taking the prepared 1,1,1,3-tetrachloropropane and anhydrous hydrogen fluoride as a raw material, and preparing 1,1,1-trifluoro-3-chloropropane in a reactor under the action of the catalyst and a polymerization inhibitor, wherein the molar ratio of 1,1,1,3-tetrachloropropane and anhydrous hydrogen fluoride is (1:3)-(1:50), the reaction pressure is 0.2-0.7MPa, the reaction temperature is 40-70DEG C, and the reaction time is 6-8 hours; and (3) carrying out dehydrochlorination on the prepared 1,1,1-trifluoro-3-chloropropane so as to prepare 3,3,3-trifluoropropene.
Owner:衢州环新氟材料有限公司
A kind of preparation method of 3,3,3-trifluoropropene
ActiveCN103044191BLow costLow reaction temperaturePreparation by hydrogen halide split-offHydrogen fluoridePtru catalyst
Owner:衢州环新氟材料有限公司
Process for the production of chlorinated propanes
InactiveUS20160002127A1Short amount of timeShorten the timePreparation by hydrogen halide split-offProduction rateAluminium chloride
Processes for the production of chlorinated propanes are provided. The processes comprise catalyzing the chlorination of 1,1,1,3-tetrachloropropane with aluminum chloride, either alone or in combination with ferric chloride. Low intensity conditions are appropriate for the process, e.g., temperatures of from ambient to 100° C. and pressures of from ambient to 200 psig may be used. Even though low intensity conditions are used, the aluminum chloride provides at least 1.5 times greater the conversion rate and / or productivity of 1,1,1,3-tetrachloropropane as compared to ferric chloride when used as a single catalyst under similar processing conditions.
Owner:BLUE CUBE IP
Catalyst for synthesizing 2-chloro-3,3,3-trifluoropropene from 1,1,2,3-tetrachloropropylene and preparation method thereof
ActiveCN102614901APhysical/chemical process catalystsPreparation by halogen replacementSucroseEthyl Chloride
The invention relates to a catalyst for synthesizing 2-chloro-3,3,3-trifluoropropene from 1,1,2,3-tetrachloropropylene and a preparation method thereof. The catalyst is composed of Cr, Al and M fluoride, wherein M is one or both of Zn and Co; the Cr-Al-M mol ratio is 1:(0.05-0.2):(0.02-0.1). The preparation method comprises the following steps: adding a KOH alkaline solution into proportional Cr compound, Al compound and M compound to form a precipitate, or directly mechanically blending the three compounds to form a mixture; adding a sucrose-citric acid solution (the sucrose-citric acid mol ratio is 1:1) into the mixture, thoroughly mixing, drying, and roasting in an N2 atmosphere to obtain an oxide containing a carbon template; and treating the oxide precursor containing carbon template with inert gas and anhydrous hydrogen fluoride, and oxidizing in an oxygen atmosphere to remove the template, thereby obtaining the catalyst. The catalyst provided by the invention has the advantages of simple technique, high activity, high selectivity, high stability and the like, and is convenient to produce.
Owner:ZHEJIANG SANMEI CHEM IND
Process for the production of chlorinated propanes
Processes for the production of chlorinated propanes are provided. The processes comprise catalyzing the chlorination of 1,1,1,3-tetrachloropropane with aluminum chloride, either alone or in combination with ferric chloride. Low intensity conditions are appropriate for the process, e.g., temperatures of from ambient to 100°C and pressures of from ambient to 200 psig may be used. Even though low intensity conditions are used, the aluminum chloride provides at least 1.5 times greater the conversion rate and / or productivity of 1,1,1,3-tetrachloropropane as compared to ferric chloride when used as a single catalyst under similar processing conditions.
Owner:BLUE CUBE IP
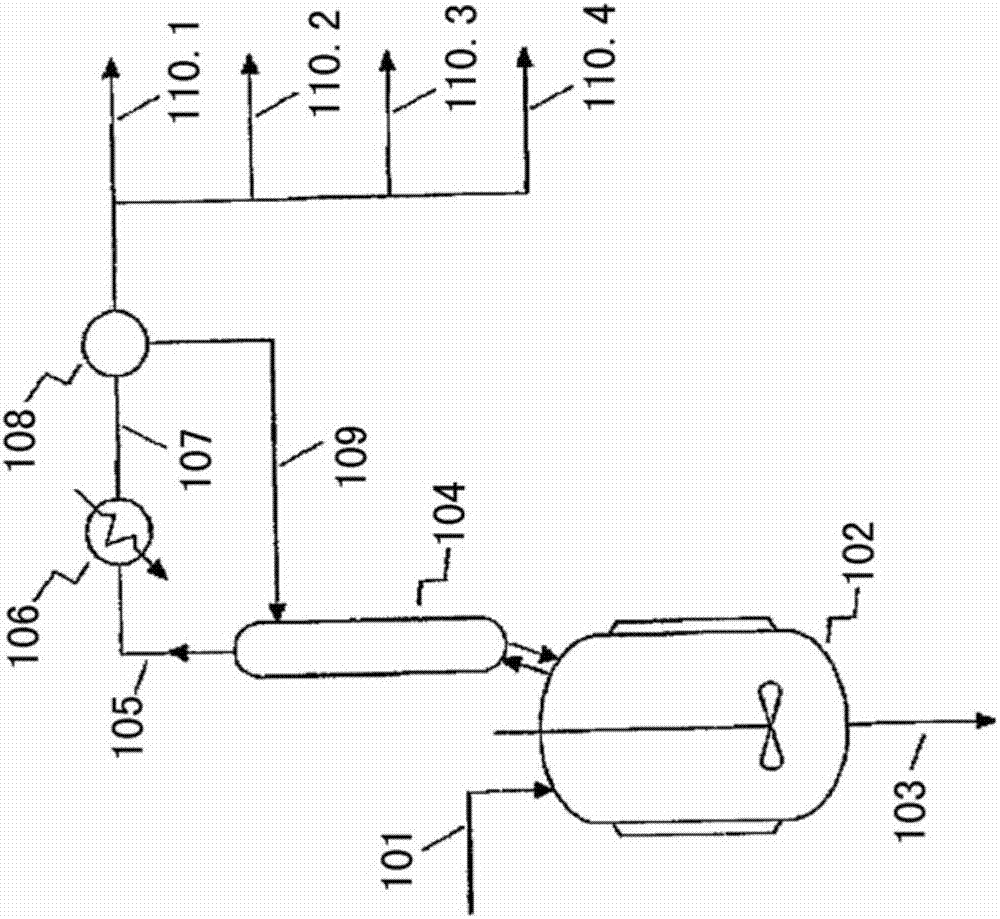
Continuous production method of 1,1,1,3-tetrachloropropane
PendingCN111056913ASimple processAchieve serializationHalogenated hydrocarbon preparationPtru catalystIndustrial engineering
The invention discloses a continuous production method of 1,1,1,3-tetrachloropropane, wherein the method comprises the steps: (1) under the action of a main catalyst, preheating a mixture of carbon tetrachloride and a cocatalyst, and simultaneously and continuously introducing the preheated mixture and ethylene into a reactor for telomerization reaction to obtain a reaction product; (2) feeding the reaction product obtained in the step (1) into a first vacuum distillation tower, and separating to obtain tower top fraction and tower bottom liquid; and (3) separating the tower bottom liquid obtained in the step (2) in a second vacuum distillation tower to obtain a 1,1,1,3-tetrachloropropane product and a high-boiling residue. The method has the advantages that the process is simple and environmentally friendly, the catalyst does not need to be independently activated, and totally-enclosed integrated continuous production is achieved.
Owner:NINGBO JUHUA CHEM TECH CO LTD
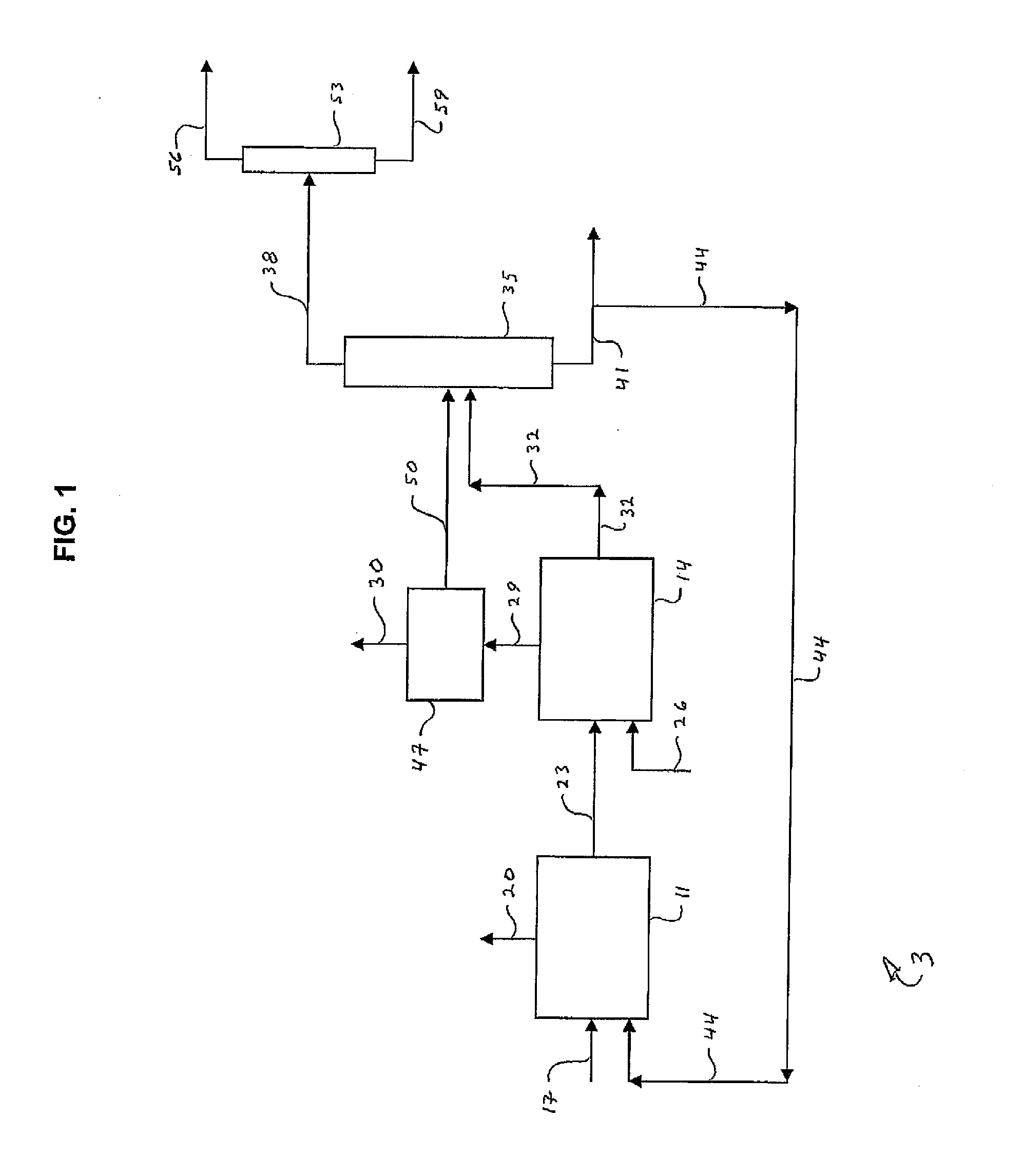
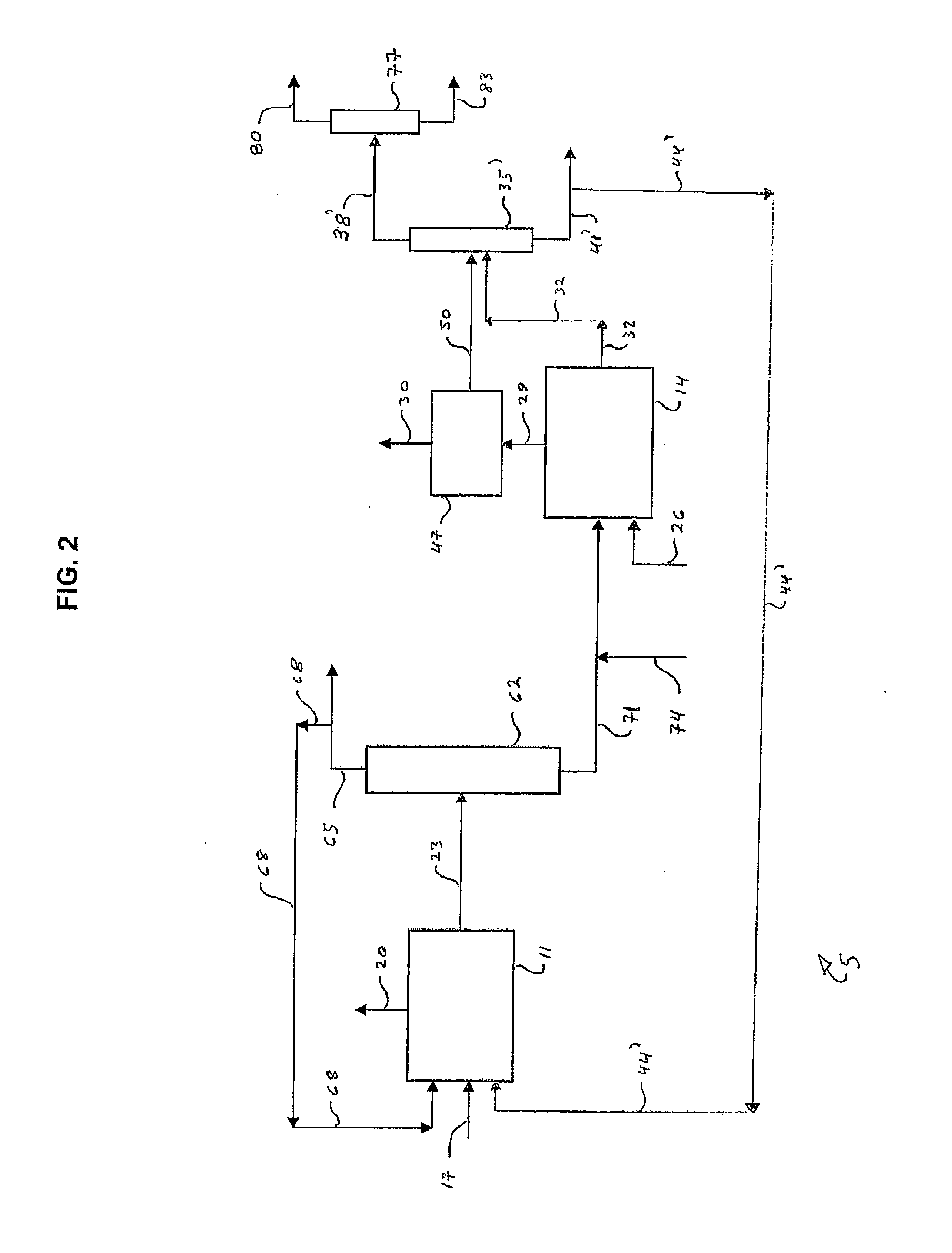
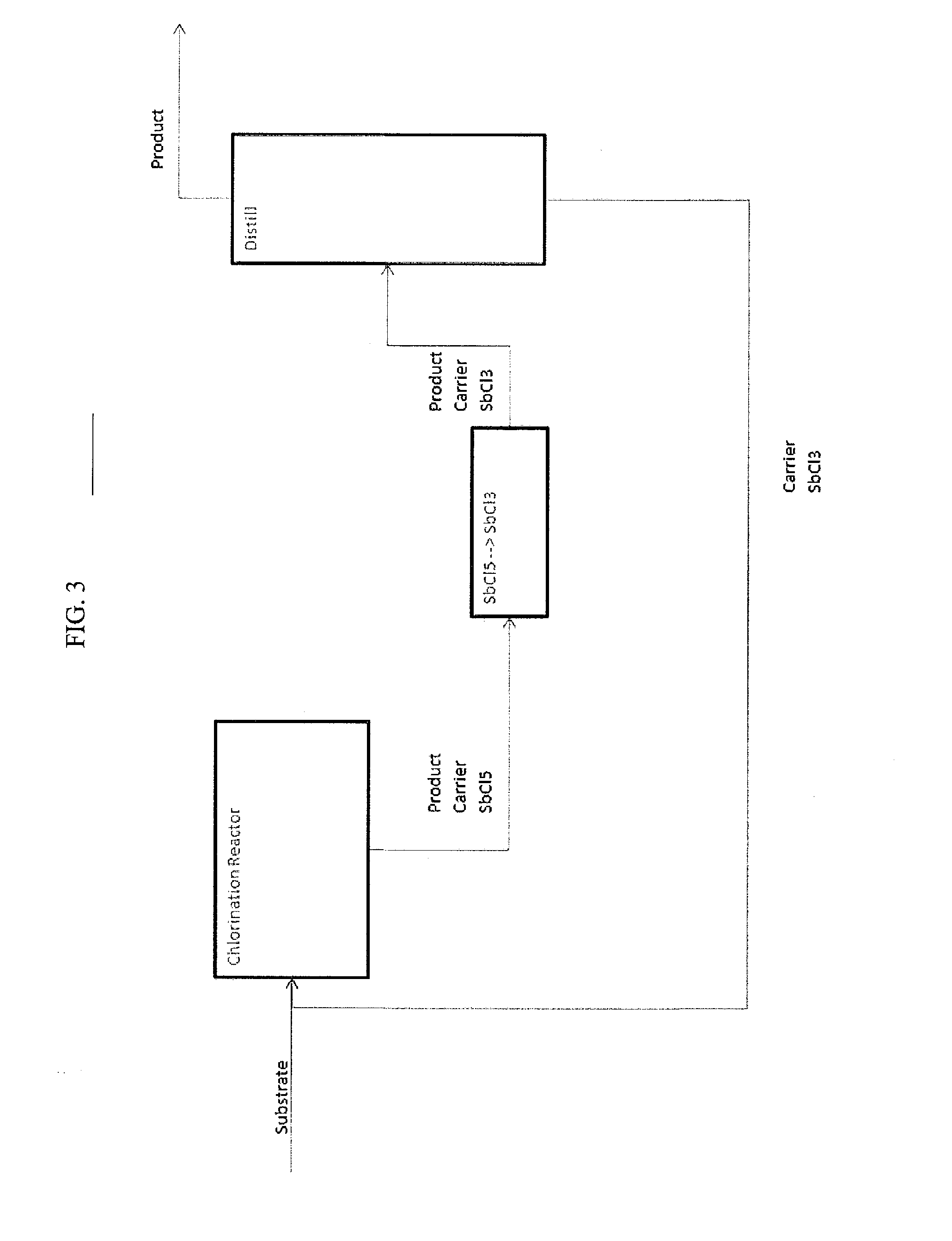
Processes for Producing Chlorinated Hydrocarbons and Methods for Recovering Polyvalent Antimony Catalysts Therefrom
ActiveUS20150045591A1Preparation by dehalogenationPreparation by hydrogen halide split-offAlkanePtru catalyst
The preparation of chlorinated hydrocarbons, such as pentachloropropanes, such as 1,1,1,2,3-pentachloropropane, from tetrachloropropanes, such as 1,1,1,3-tetrachloropropane, in the presence of a polyvalent antimony compound that includes a pentavalent antimony compound, such as antimony pentachloride, is described. Also described are methods for preparing optionally chlorinated alkenes, such as, tetrachloropropenes, from chlorinated alkanes, such as pentachloropropanes, in the presence of polyvalent antimony compound that includes a pentavalent antimony compound, as well as methods for recovering polyvalent antimony compounds from such processes.
Owner:EAGLE US 2 LLC
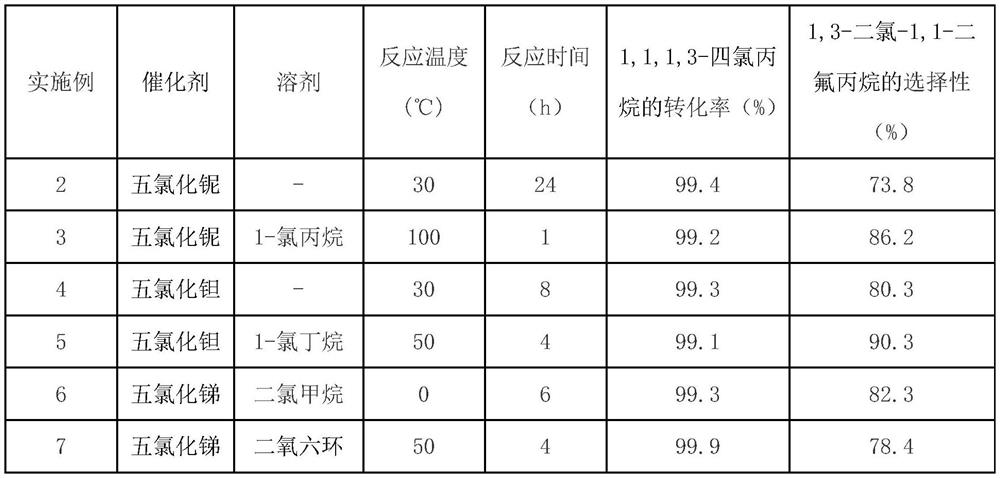
Preparation method of 1, 3-dichloro-1, 1-difluoropropane
ActiveCN114213211ARaw materials are easy to getImprove conversion ratePreparation by halogen replacementPtru catalystAntimony pentachloride
The invention discloses a preparation method of 1, 3-dichloro-1, 1-difluoropropane, and belongs to the technical field of fluorine-containing chemicals. Under the action of a lewis acid catalyst, in the presence or absence of a solvent, 1, 1, 1, 3-tetrachloropropane reacts with antimony trifluoride to generate 1, 3-dichloro-1, 1-difluoropropane, and the lewis acid catalyst is niobium pentachloride, tantalum pentachloride or antimony pentachloride. According to the preparation method of the 1, 3-dichloro-1, 1-difluoropropane, the raw materials are easy to obtain, the conversion rate is high, the 1, 3-dichloro-1, 1-difluoropropane can be prepared with high selectivity, and the selectivity can reach 90% under better conditions.
Owner:ZHEJIANG FLUORINE CHEM NEW MATERIAL
Method for synthesizing 2,3,3,3-tetrafluoropropene
ActiveCN104987278AWide variety of sourcesReduce manufacturing costPreparation by halogen replacementDichloropropane1,2-Dichloropropane
The invention discloses a method for synthesizing 2,3,3,3-tetrafluoropropene and belongs to the field of organic synthesis. The method comprises the following steps: (1) preparing 2-chloropropene from 1,2-dichloropropane, which serves as a raw material, through continuous catalytic cracking by adopting a fixed bed in the presence of beta-zeolite, which serves as a catalyst; (2) selectively chlorinating 2-chloropropene with chlorine gas under the catalysis of ferric chloride, so as to prepare 2,3,3,3-tetrachloropropylene; and (3) fluorating 2,3,3,3-tetrachloropropylene with hydrofluoric acid under the catalysis of SbF3 or SbF5, thereby obtaining 2,3,3,3-tetrafluoropropene. The synthesis route has the advantages that the source of raw materials is wide, the cost is low, and the product yield is high; and the obtained product can serve as an automotive air conditioning refrigerant and has a positive significance in reduction of greenhouse effect.
Owner:山东联创产业发展集团股份有限公司
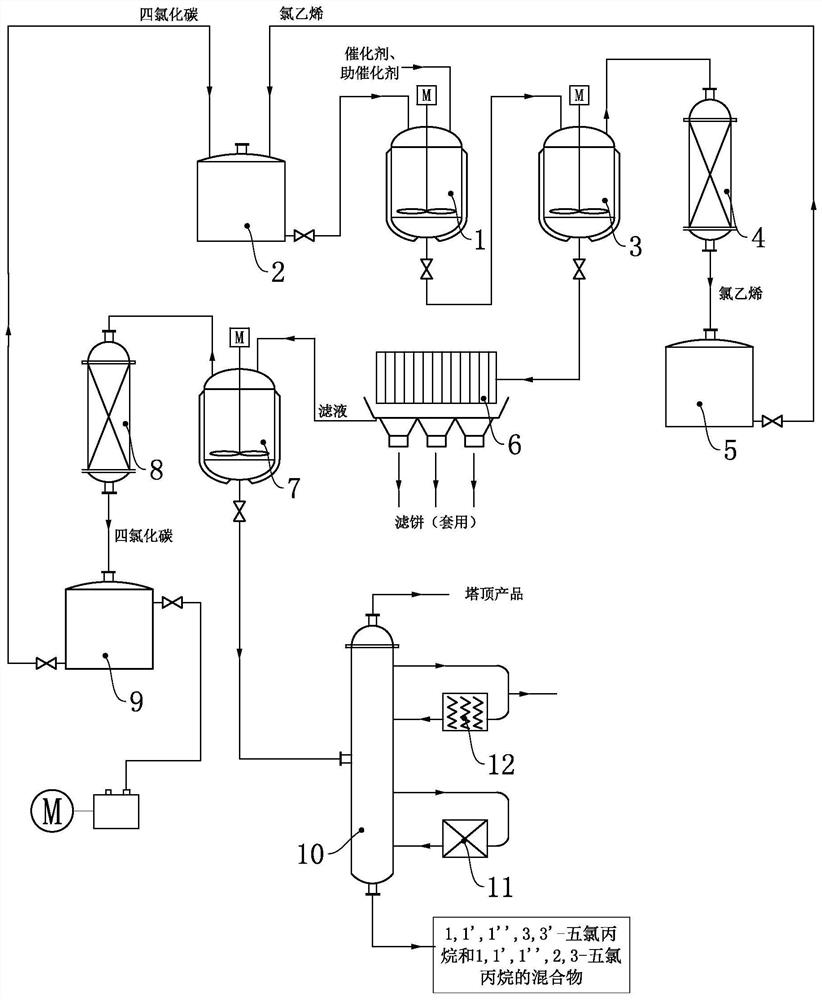
Co-production method and device for 3, 3, 3-trifluoropropene and 2-chloro-3, 3, 3-trifluoropropene
ActiveCN112537997AEfficient separationImprove the mixing effectPreparation by hydrogen halide split-offPreparation by halogen replacementEngineeringIndustrial engineering
The invention relates to a co-production method and device for 3, 3, 3-trifluoropropene and 2-chloro-3, 3, 3-trifluoropropene. The device used in the method comprises a reaction system, a rectification pre-separation system and an azeotrope-like substance extraction separation system. The raw material premixer adopts a falling film evaporation premixer, so that the liquid materials 1, 1, 1, 3-tetrachloropropane are promoted to be quickly and fully vaporized, and meanwhile, the mixing effect of each gas-phase material is enhanced. The rectification pre-separation system adopts a three-tower continuous rectification mode to realize efficient separation of reaction by-products hydrogen chloride, high-boiling residues and excessive hydrogen fluoride. And the azeotrope-like substance extractionand separation system is used for realizing liquid-liquid two-phase extraction and separation of azeotrope-like substances. The production method provided by the invention is realized through the production device with the specific structure and connection relationship, has the advantages of mild reaction conditions, high reaction rate, good atom economy, stable product quality, adjustable product proportion and the like, can realize full-flow automatic control, saves labor, and is suitable for industrial co-production of 3, 3, 3-trifluoropropene and 2-chloro-3, 3, 3-tetrafluoropropene.
Owner:新元化学(山东)股份有限公司 +1
A kind of preparation method of 1,1,1,3-tetrachloropropane
ActiveCN111470938BReduce decompositionIncrease profitPhysical/chemical process catalystsCatalystsPtru catalystCarbon Chloride
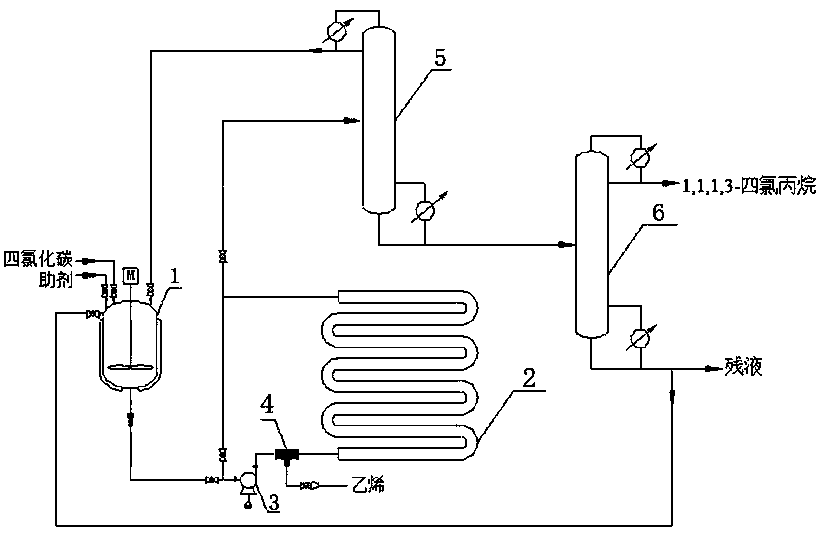
The invention discloses a preparation method of 1,1,1,3-tetrachloropropane. Carbon tetrachloride and an auxiliary agent are mixed, preheated and forced to circulate for a period of time, and then reacted with ethylene in an intensified reactor for forced circulation. The crude product of 1,1,1,3-tetrachloropropane was obtained, which was finally separated by vacuum distillation to obtain 1,1,1,3-tetrachloropropane. The method for preparing 1,1,1,3-tetrachloropropane provided by the present invention has simple process, low energy consumption, and easy control of the reaction process. 75% to 95% of the residual liquid in the second rectification column can be recycled, with high material utilization rate, less waste generation, and high purity of the target product. At the same time, the use of ammonia-nitrogen-free and phosphorus-free catalysts solves the problem of nitrogen and phosphorus. The pollution of the three wastes is highly efficient and environmentally friendly.
Owner:常州新东化工发展有限公司
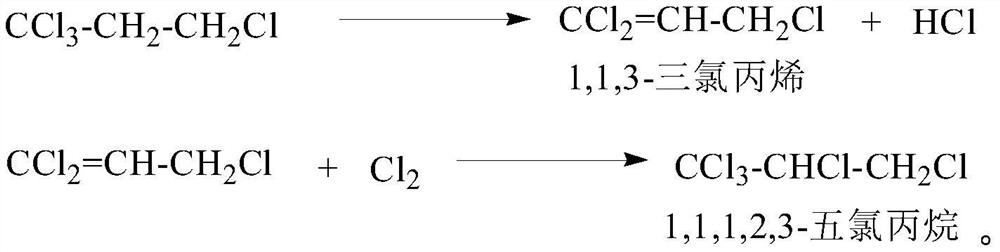
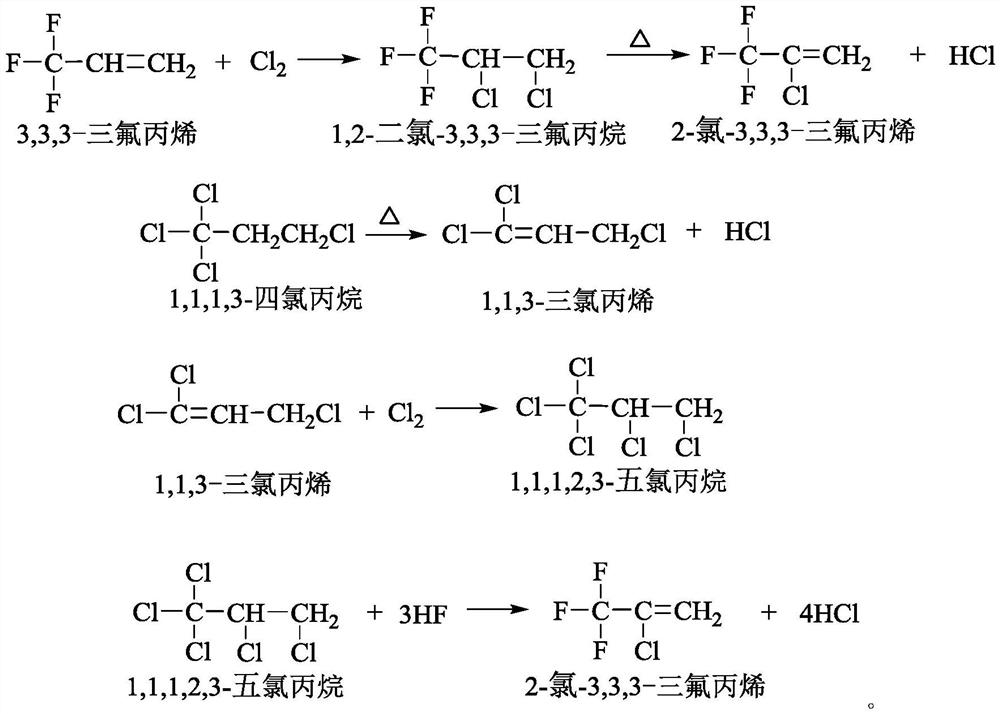
Method for producing 1,1,3-trichloropropene by liquid-phase dehydrochlorination of 1,1,1,3-tetrachloropropane and recovering catalyst
ActiveCN111559952AHigh boiling lessSolve the problem of difficult separation and reusePreparation by hydrogen halide split-offOrganic compound preparationHomogeneous catalysisTetrachloropropylene
The invention discloses a method for producing 1,1,3-trichloropropene by liquid-phase homogeneous dehydrochlorination of 1,1,1,3-tetrachloropropane. The method comprises the following steps: using iron p-toluenesulfonate as a catalyst, carrying out heating dehydrochlorination on 1,1,1,3-tetrachloropropane in a stirred tank reactor to produce 1,1,3-trichloropropene, and adding an extractant after the reaction to recover the catalyst iron p-toluenesulfonate. The catalyst is good in activity, high in selectivity, easy to separate and recycle and environmentally friendly, after the reaction is finished, the catalyst is extracted and recycled through a solvent, waste solid emission is reduced, and the activity of the recycled catalyst is kept unchanged.
Owner:常州新东化工发展有限公司
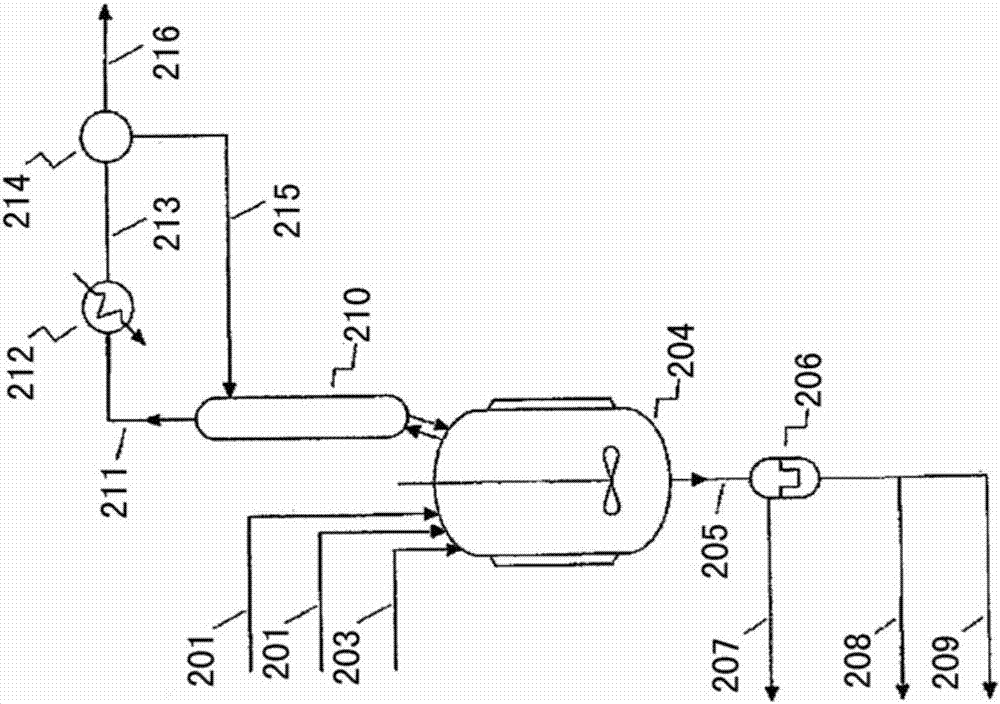
Device and process for preparing 1,1,1,3-tetrachloropropane
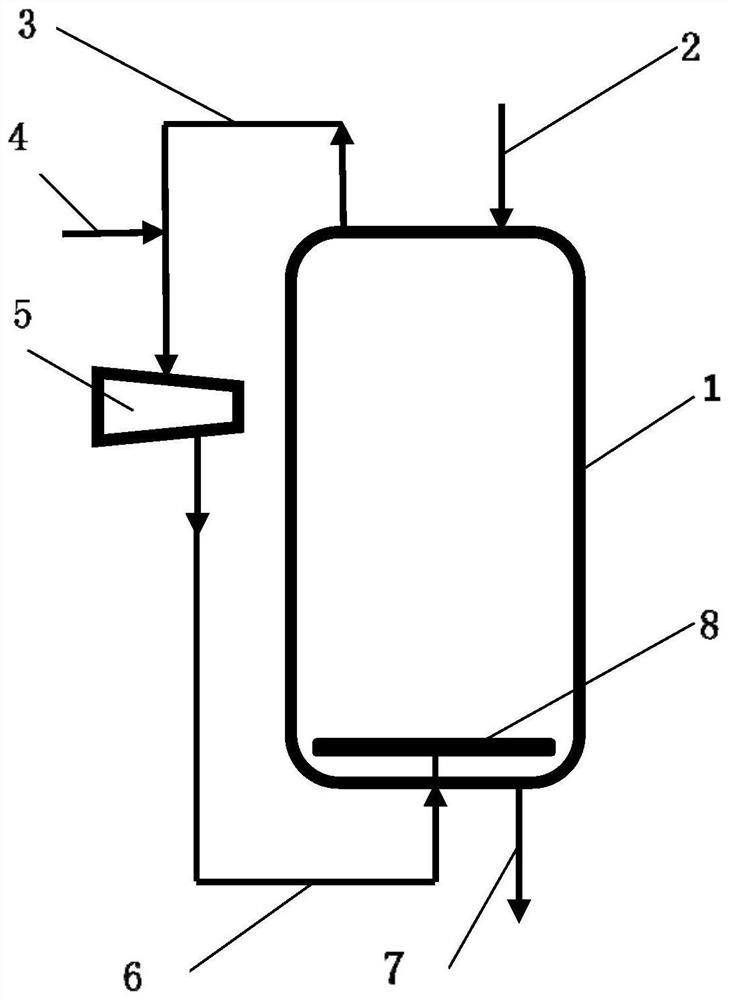
PendingCN111659322AReduce manufacturing costStir wellHalogenated hydrocarbon preparationChemical/physical processesPolymer scienceCarbon tetrachloride
The invention discloses a reaction device for preparing 1,1,1,3-tetrachloropropane. The reaction device comprises a reaction kettle, wherein an ethylene circulating system is arranged outside the reaction kettle, a gas distributor is arranged at the bottom of the reaction kettle, the ethylene circulating system comprises an ethylene extraction pipe arranged at the upper portion of the reaction kettle, an ethylene press-in pipe arranged at the lower portion of the reaction kettle, and an ethylene compressor arranged between the ethylene extraction pipe and the press-in pipe, and the ethylene press-in pipe is communicated with the gas distributor. The invention also discloses a process for preparing 1,1,1,3-tetrachloropropane by utilizing the reaction device to react carbon tetrachloride with ethylene. By adopting the process provided by the invention, the generation amount of colloidal oily by-products in the process of preparing 1,1,1,3-tetrachloropropane by reacting carbon tetrachloride with ethylene is reduced, the selectivity of the main product 1,1,1,3-tetrachloropropane is improved, the reaction kettle does not need a stirring device, and the pressure of ethylene in the reaction kettle is reduced at a similar reaction rate.
Owner:浙江佳汇新材料有限公司 +1
Preparation method of 1,1,1,3-tetrachloropropane
ActiveCN111470938AReduce decompositionIncrease profitPhysical/chemical process catalystsCatalystsPtru catalystCarbon Chloride
The invention discloses a preparation method of 1,1,1,3-tetrachloropropane, which comprises the following steps: mixing carbon tetrachloride with an assistant, preheating, carrying out forced circulation for a period of time, carrying out forced circulation reaction with ethylene in an enhanced reactor to obtain a 1,1,1,3-tetrachloropropane crude product, and carrying out vacuum distillation separation to obtain 1,1,1,3-tetrachloropropane. The method is simple in process and low in energy consumption; the reaction process is easy to control; ethylene, carbon tetrachloride and the assistant obtained at the tower top of the first rectifying tower and 75-95% of residual liquid in the tower bottom of the second rectifying tower can be recycled, the material utilization rate is high, less wasteis generated, the purity of the prepared target product is high, meanwhile, ammonia-nitrogen-free and phosphorus-free catalysts are used, the pollution problem of nitrogen-containing and phosphorus-containing three wastes is solved, and high efficiency and environmental protection are achieved.
Owner:常州新东化工发展有限公司
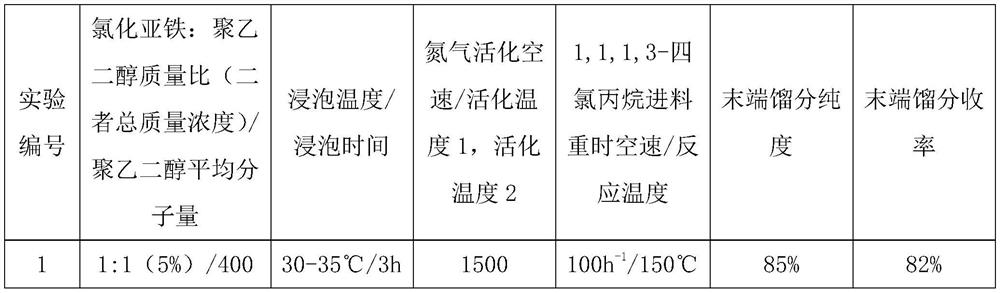
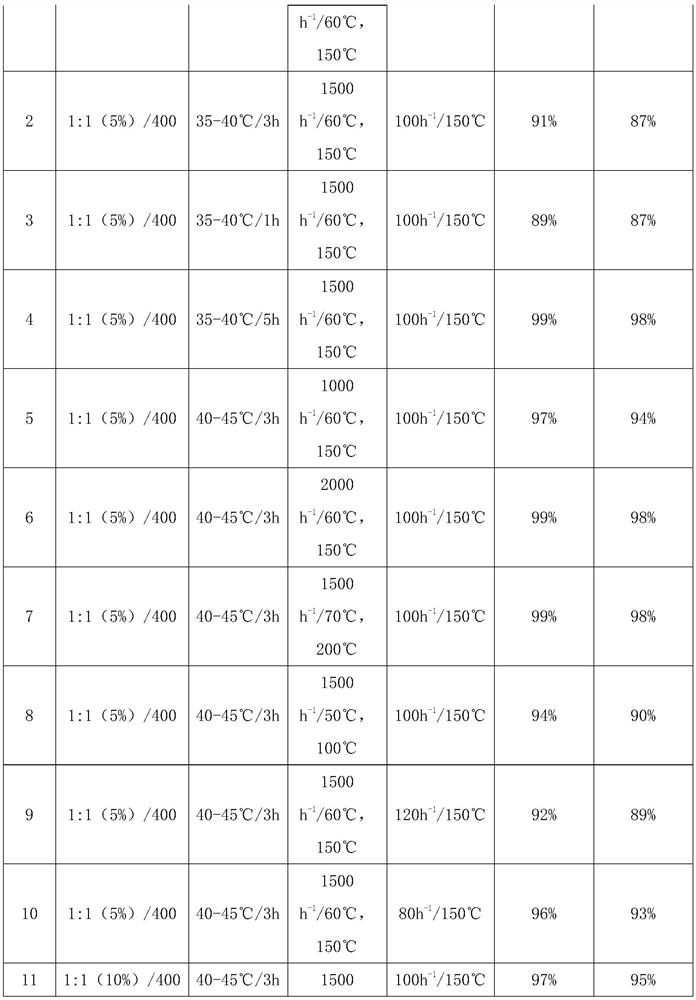
Gamma-aluminum oxide modified catalyst, preparation method thereof and application of gamma-aluminum oxide modified catalyst in synthesis of 1, 1, 3-trichloropropene
ActiveCN111604041ASimple preparation processImprove efficiencyCatalyst carriersPreparation by hydrogen halide split-offPtru catalystOrganic synthesis
The invention discloses a gamma-aluminum oxide modified catalyst, a preparation method thereof and application of the gamma-aluminum oxide modified catalyst in synthesis of 1, 1, 3-trichloropropene, and belongs to the field of catalyst preparation and organic synthesis. The catalyst takes gamma-aluminum oxide as a carrier and is modified by ferrous chloride and polyethylene glycol to obtain the gamma-aluminum oxide modified catalyst. The application method comprises the following steps: loading the modified catalyst into a tubular reactor, carrying out nitrogen gas activation, under heating condition, introducing 1, 1, 1, 3-tetrachloropropane continuously, so that continuously obtaining of 1, 1, 3-trichloropropene at the tail end of a reaction tube is realized. The modified gamma-aluminumoxide catalyst is used for preparing 1, 1, 3-trichloropropene from 1, 1, 1, 3-tetrachloropropane, has the advantages of being high in efficiency, good in stability and capable of being continuously used for a long time, and has the potential of further enlarged production.
Owner:DALIAN JOIN KING FINE CHEM CO LTD
A kind of 1,1,1,3-tetrachloropropane liquid-phase dehydrochlorination to produce 1,1,3-trichloropropene and catalyst recovery method
ActiveCN111559952BHigh boiling lessSolve the problem of difficult separation and reusePreparation by hydrogen halide split-offOrganic compound preparationPtru catalystFluid phase
The invention discloses a method for producing 1,1,3-trichloropropene by liquid-phase homogeneous catalyzed dehydrochlorination of 1,1,1,3-tetrachloropropane. In a tank reactor, 1,1,1,3-tetrachloropropane is heated and dehydrochlorinated to produce 1,1,3-trichloropropene, and after the reaction, an extractant is added to recover the catalyst iron p-toluenesulfonate. The catalyst of the invention has good activity, high selectivity, easy separation and reuse, and is environmentally friendly. After the reaction is completed, the catalyst is extracted and recovered by using a solvent, reducing waste solid discharge, and the activity of the recovered catalyst remains unchanged.
Owner:常州新东化工发展有限公司
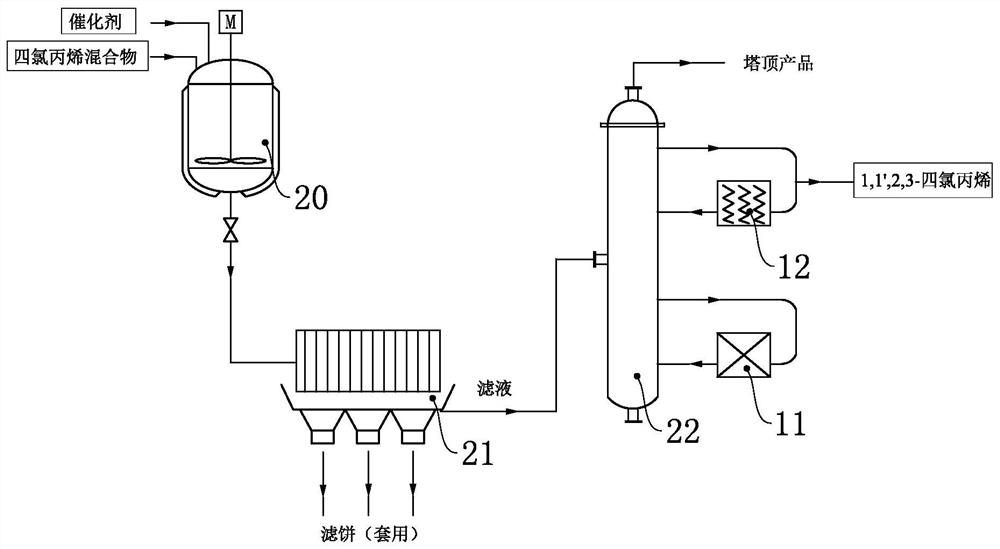
A method and equipment for clean and environmentally friendly production of 1,1',2,3-tetrachloropropene
ActiveCN108033872BSmooth responseSolve pollutionPreparation by hydrogen halide split-offPtru catalystReaction temperature
The invention discloses a clean and environment-friendly method for producing 1,1',2,3-tetrachloropropene, comprising the following steps: under the premise of nitrogen pressure protection, adding carbon tetrachloride, chlorine Ethylene, and use the catalyst, co-catalyst to catalyze the reaction to obtain the mixture; put the above mixture in the No. With the mol ratio of nitrogen, catalytic reaction obtains tetrachloropropene mixture; With above-mentioned tetrachloropropene mixture, in No. three autoclaves, with FeFeCl 3 As a catalyst, the product 1,1',2,3-tetrachloropropene is obtained by controlling the reaction temperature. The invention also discloses special equipment. The technological process of the present invention is simple, the equipment requirement is relatively low, and is beneficial to industrialization promotion.
Owner:安庆市华璞环保材料科技有限责任公司
One-pot method for preparing 1,1,1,2,3-pentachloropropane with high selectivity and high yield
ActiveCN108069817BHigh yieldPreparation by hydrogen halide split-offPreparation by halogen additionPhysical chemistrySide reaction
The invention provides a method for preparing 1,1,1,2,3-pentachloropropane with high selectivity and high yield in a one-pot method. The method uses 1,1,1,3-tetrachloropropane as a raw material to remove Hydrogen chloride reaction to obtain 1,1,3-trichloropropene, when the content of 1,1,3-trichloropropene in the system reaches 4-20%, feed chlorine to react, after feeding chlorine, control 1,1, The content of 3-trichloropropene is 1-6%. When the content of 1,1,1,2,3-pentachloropropane generated in the system reaches 90-99%, stop the reaction. The present invention utilizes a one-pot method to prepare 1,1,1,2,3-pentachloropropane, by controlling the content of 1,1,3-trichloropropene in the system after the dehydrochlorination reaction and introducing chlorine gas, and controlling the generated The control of the content of 1,1,1,2,3-pentachloropropane can greatly reduce side reactions and improve the selectivity and yield of preparing 1,1,1,2,3-pentachloropropane.
Owner:JIANGXI TIANYU CHEM CO LTD
The synthetic method of 2,3,3,3-tetrafluoropropene
ActiveCN104987278BWide variety of sourcesReduce manufacturing costPreparation by halogen replacementDichloropropaneOrganic synthesis
Owner:山东联创产业发展集团股份有限公司
A method for continuously synthesizing 2,3,3,3-tetrafluoropropene in gas phase
ActiveCN109438171BHigh yieldReaction conditions are easy to controlPreparation by hydrogen halide split-offPreparation by halogen halide additionHydrogen halidePtru catalyst
The invention discloses a method for continuously synthesizing 2,3,3,3-tetrafluoropropene in a gas phase. In the presence of a catalyst, tetrachloroethylene and methyl chloride undergo a telomerization reaction to generate 1,1,1,2, Catalyzed fluorination of 2‑pentachloropropane and 2,3,3,3‑tetrachloropropene, ,1,1,2,2‑pentachloropropane and 2,3,3,3‑tetrachloropropene to 1,1 ,1,2,2-Pentafluoropropane and 2-Chloro-1,1,1,2-Tetrafluoropropane, 1,1,1,2,2-Pentafluoropropane and 2-Chloro-1,1,1 ,2-Tetrafluoropropane Catalytic Dehydrohalogenation to Prepare 2,3,3,3-Tetrafluoropropene. The invention adopts a continuous method for reaction, the raw materials are economical and easy to obtain, and the reactions of each step are carried out in a reactor, the reaction conditions are easy to control, and the equipment operation is simple; and the conversion rate of each step of the preparation method is better, and the yield of the target product is higher , low equipment investment cost and other advantages, has a wide range of industrial application prospects.
Owner:ZHEJIANG SANMEI CHEM IND
Process for Preparing R-1234yf by Base Mediated Dehydrohalogenation
ActiveUS20130217927A1Preparation by hydrogen halide split-offOrganic compound preparationPolymer scienceDehydrohalogenation
The invention relates to a process for preparing 2,3,3,3-tetrafluoropropene (CF3CF═CH2), performed using the steps of dehydrohalogenating 1,1,1,2,2-pentafluoropropane (CH3CF2CF3, HFC-245ca) 1,1,1,2-tetrafluoro-2-chloropropane, 1,1,1,2,3-pentafluoropropane (CH2FCHFCF3, HFC-245eb) and / or 1,1,1,2-tetrafluoro-3-chloropropane in the presence of a base, and converting a trifluorodichloropropane or a difluorotrichloropropane or a fluorotetrachloropropane to CH3CF2CF3, 1,1,1,2-tetrafluoro-2-chloropropane, CH2FCHFCF3, and / or 1,1,1,2-tetrafluoro-3-chloropropane.
Owner:MEXICHEM AMANCO HLDG DE C V
Compositions and methods for an integrated 2,3,3,3-tetrafluoropropene manufacturing process
PendingUS20210198168A1High selectivityHigh yieldPreparation by dehalogenationPreparation by halogen replacementHydrogen fluoridePtru catalyst
A method of synthesizing 3,3,3-trifluoropropene including contacting 1,3,3,3-tetrachloropropane, in the vapor phase, at a temperature sufficient to effect dehydrochlorination to form 1,1,3-trichloro-1-propene. The 1,1,3-trichloro-1-propene is isolated and subsequently contacted, in the vapor phase, with hydrogen fluoride in the presence of a fluorination catalyst at a temperature sufficient to effect formation of 3,3,3-trifluoropropene.
Owner:THE CHEMOURS CO FC LLC
A kind of preparation method of 3,3,3-trifluoropropene
ActiveCN108610233BAdequate responseEasy to getPreparation by hydrogen halide split-offPreparation by halogen replacementHydrogen fluoridePtru catalyst
The invention discloses a preparation method of 3,3,3-trifluoropropene, (1) uniformly mixing 1,3,3,3-tetrachloropropane and an inhibitor at a molar ratio of 1:5-50; (2 ) Send the mixture to the first vaporizer for vaporization, the vaporization temperature is 80°C-300°C; (3) Pass hydrogen fluoride into the second vaporizer for vaporization, the vaporization temperature is 80°C-300°C; (4) After the steps ( The product of 2) and the product of step (3) are simultaneously fed into a gas phase reactor equipped with a catalyst for reaction, the total molar ratio of 1,3,3,3-tetrachloropropane and inhibitor to hydrogen fluoride is 1:1, Reaction temperature 200℃~600℃, reaction space velocity 2000h ‑1 ~4000h ‑1 , the reaction pressure is 0.2Mpa-1.0Mpa; (5) Purifying the product in step (4) to obtain 3,3,3-trifluoropropene. The invention improves the service life of the catalyst, reduces impurities produced in the preparation process, and improves the conversion rate of 1,3,3,3-tetrachloropropane and the yield of 3,3,3-trifluoropropene.
Owner:衢州环新氟材料有限公司
A kind of gas phase preparation method of 1,1,2,3-tetrachloropropene
ActiveCN109134190BHigh activitySolution to short lifePreparation by hydrogen halide split-offHalogenated hydrocarbon separation/purificationPtru catalystReaction temperature
The invention discloses a gas-phase preparation method of 1,1,2,3-tetrachloropropene. Under the action of a catalyst, 1,1,1,2,3-pentachloropropane undergoes a gas-phase catalytic dehydrochlorination reaction, and the reaction The temperature is 150-250° C., the contact time is 1-15 seconds, and the reaction product is collected and rectified under reduced pressure to obtain 1,1,2,3-tetrachloropropene product. The invention has the advantages of simple process, good catalyst activity, long service life, environmental protection and easy industrialization.
Owner:JUHUA GROUP TECH CENT
Compositions and methods for an integrated 2,3,3,3-tetrafluoropropene manufacturing process
PendingCN112384490AGood choiceHigh yieldPreparation by dehalogenationPreparation by halogen replacementHydrogen fluoridePtru catalyst
A method of synthesizing 3,3,3-trifluoropropene including contacting 1,3,3,3-tetrachloropropane, in the vapor phase, at a temperature sufficient to effect dehydrochlorination to form 1,1,3-trichloro-1-propene. The 1,1,3-trichloro-1-propene is isolated and subsequently contacted, in the vapor phase, with hydrogen fluoride in the presence of a fluorination catalyst at a temperature sufficient to effect formation of 3,3,3-trifluoropropene.
Owner:THE CHEMOURS CO FC LLC
Synthetic method of cis-trans 1-chloro-3,3,3-trifluoropropene
ActiveCN105152850BThe synthesis method is simpleLow costPreparation by halogen replacementHydrogen fluorideIsomerization
The invention relates to a method for synthesizing cis / trans-3,3,3-trifluoro-1-chloropropylene and belongs to the technical field of organic synthesis. The method comprises the following steps: (1) selectively chlorinating 1,3-dichloropropylene by chlorine gas in the presence of a catalyst, i.e., ferric chloride, so as to synthesize 1,3,3,3-tetrachloropropylene; and (2) fluorating 1,3,3,3-tetrachloropropylene by hydrogen fluoride in the presence of a catalyst, i.e., antimony pentafluoride, thereby synthesizing cis / trans-3,3,3-trifluoro-1-chloropropylene. The product cis / trans-3,3,3-trifluoro-1-chloropropylene can be used as a polyurethane foaming agent, and trans-3,3,3-trifluoro-1-chloropropylene can be used for replacing HCFC, HFC and alkane foaming agents, is an ideal environment-friendly polyurethane foaming agent and can be prepared from cis isomers through ionic liquid catalysis or ultraviolet illumination isomerization.
Owner:山东联创聚合物有限公司
Preparation method of 1, 1, 1, 3-tetrachloropropane
PendingCN114835554AEasy to removeImprove the problem of easy reunionOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationTetrachloropropylene1-Chloropropane
The invention discloses a preparation method of 1, 1, 1, 3-tetrachloropropane, which comprises the following steps: in the presence of a first catalyst and a second catalyst, reacting raw materials containing carbon tetrachloride and ethylene to obtain the 1, 1, 1, 3-tetrachloropropane, the first catalyst comprises a carrier and an active component loaded on the carrier; the active component comprises nano zero-valent iron; the second catalyst comprises an ester compound. According to the preparation method, the supported iron catalyst is adopted, so that the problem of easy agglomeration when pure commercially available iron powder is used as the catalyst can be remarkably improved, the specific surface area of iron is increased, and the reaction is facilitated.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
A kind of preparation method of 1,1,1,2,3-pentachloropropane
ActiveCN109809959BHigh yieldImprove conversion ratePreparation by dehalogenationPreparation by hydrogen halide split-offOligomerProcess engineering
Owner:JIANGXI TIANYU CHEM CO LTD
A co-production method and device for 3,3,3-trifluoropropene and 2-chloro-3,3,3-trifluoropropene
ActiveCN112537997BImprove the mixing effectFully vaporizedPreparation by hydrogen halide split-offPreparation by halogen replacementHydrogen fluorideProcess engineering
The invention relates to a co-production method and device for 3,3,3-trifluoropropene and 2-chloro-3,3,3-trifluoropropene. The device used in the method includes a reaction system, a rectification pre-separation system, There are three parts in the azeotrope-like extraction and separation system; the raw material pre-mixer adopts a falling-film evaporation pre-mixer to promote the rapid and full vaporization of the liquid material 1,1,1,3-tetrachloropropane, and at the same time, strengthen the mixing effect of each gas phase material. The rectification pre-separation system adopts the three-tower continuous rectification method to realize the efficient separation of reaction by-product hydrogen chloride, high boilers and excess hydrogen fluoride. The azeotrope-like extraction and separation system realizes the liquid-liquid two-phase extraction and separation of azeotrope-like substances. The production method provided by the present invention is realized by the production device with the above-mentioned specific structure and connection relationship, and has the advantages of mild reaction conditions, high reaction rate, good atom economy, stable product quality, adjustable product ratio, etc., and can realize automatic control of the whole process , labor-saving, suitable for industrial co-production of 3,3,3-trifluoropropene and 2-chloro-3,3,3-trifluoropropene.
Owner:新元化学(山东)股份有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com