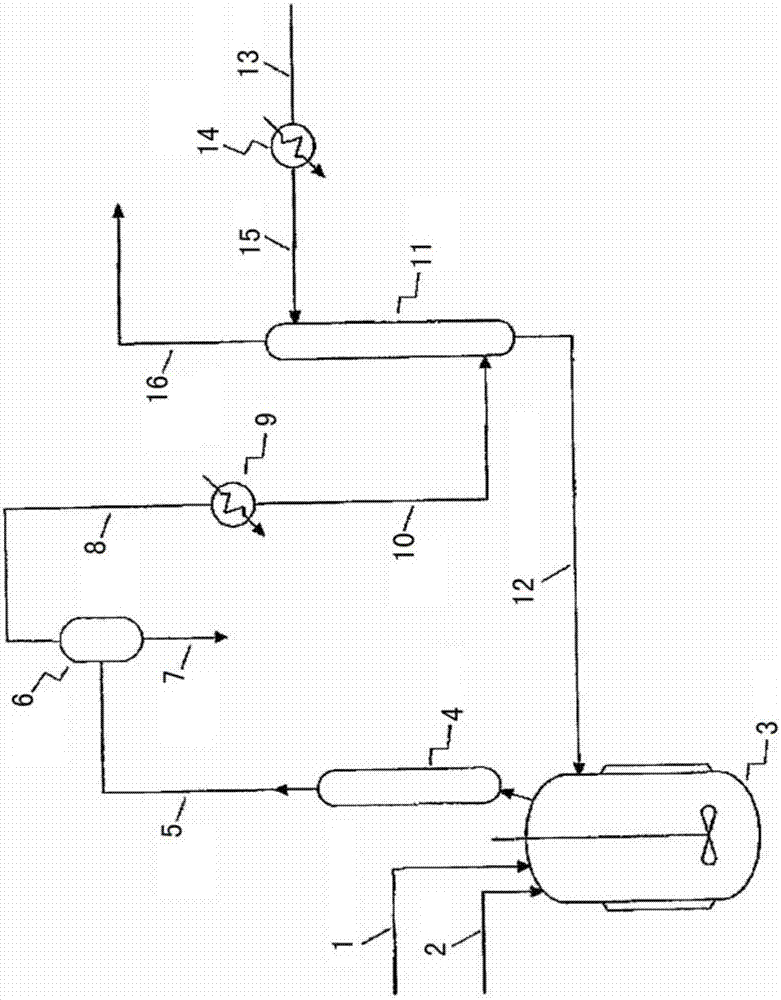
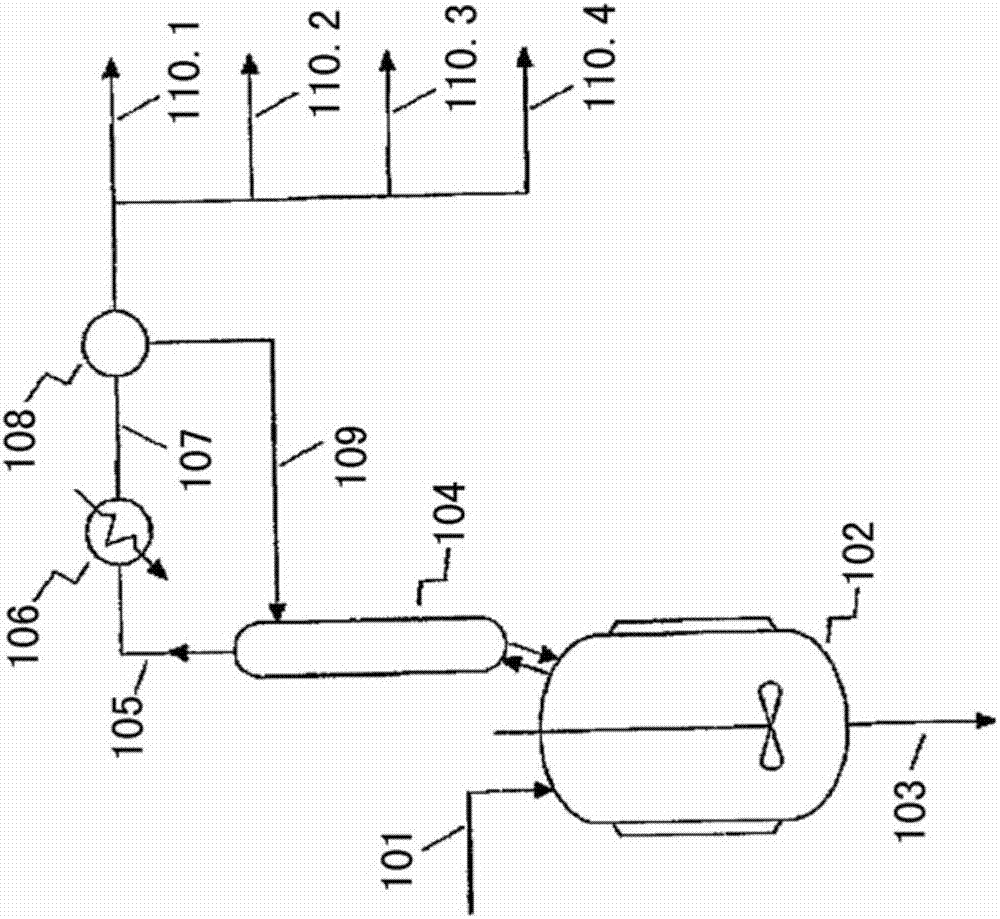
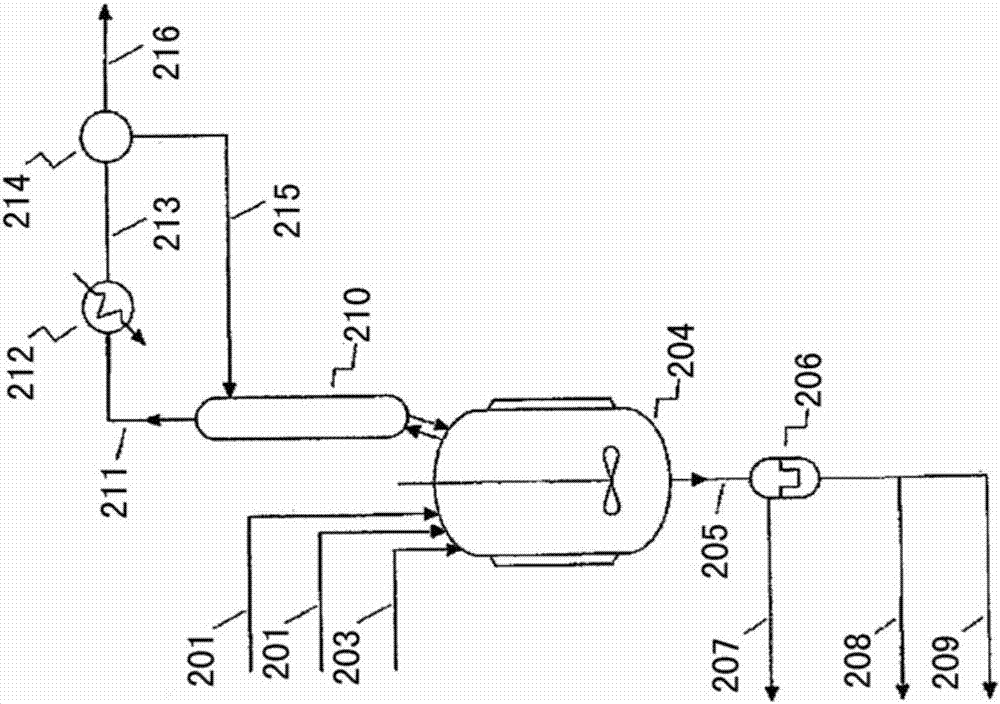
Process
A technology of reaction mixture and tetrachloropropane, applied in the field of compositions containing such compounds, can solve the problems of difficult separation, reduced catalyst effectiveness or working life, etc., and achieve the effect of continuous production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0407] Example 1 - Demonstration of Catalytic Capability of Recovered Catalyst Using Water Treatment.
[0408] Ethylene and carbon tetrachloride are reacted to produce 1,1,1,3-tetrachloropropane in the presence of a catalyst i) recovered from the reaction mixture using conventional distillation techniques, or ii) using the method described herein for The inventive water treatment step of the catalyst is recovered from the reaction mixture. The reaction mixture additionally contains 1,1,1,3-tetrachloropropane (present in the recycle stream) and tetrachloropentane (usually formed as a by-product in the presence of a telomerization reaction between carbon tetrachloride and ethylene chlorinated alkanes impurity).
[0409] These test examples show that catalyst performance is significantly higher using a water treatment step to recover catalyst compared to catalyst recovered using conventional distillation techniques.
[0410] The progress of the reaction was monitored using gas ...
Embodiment 1-2
[0418] 90.1 g containing 63.7% TeCPa, 22.8% TeCPna and 7.49% Bu 3 PO 4 The distillation residue was extracted with 370 g of 5% HCl. The bottom organic layer was filtered and mixed with 400 g TeCM. Then, the mixture was introduced into an autoclave to which 5.0 g of iron had been added. After rinsing with ethylene, the mixture was heated to 110°C in an autoclave. At this temperature and an ethylene pressure of 9 bar, the reaction mixture was allowed to react for 4.5 hours. The first sample was taken after 3 hours. The concentration of residual TeCM at the end of the experiment was 5.5% (24.6% after 3 hours).
Embodiment 1-4 and 1-5
[0422] Examples 1-4 and 1-5 were performed using the same conditions as used in Example 1-2, except that different concentrations of carbon tetrachloride and tributyl phosphate were used.
[0423] The results of Comparative Example 1-1 and Example 1-2, and Comparative Example 1-3 and Examples 1-4 and 1-5 are shown in the following table. It can be seen that in Examples 1-2, 1-4 and 1-5, tetrachloropropane converted to 1,1,1,3-tetrachloropropane The percentage of chlorinated carbons was significantly higher, suggesting that the conduct of the water treatment step has a profoundly positive effect on the system when the catalyst is recovered. This is due to the high activity of the catalyst recovered from the distillate residue, and possibly also due to the removal of impurities (such as oxygen-containing impurities) from the reaction mixture that might otherwise retard the reaction.
[0424]
[0425] Continuous configuration:
[0426] The same stainless steel autoclave as d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com