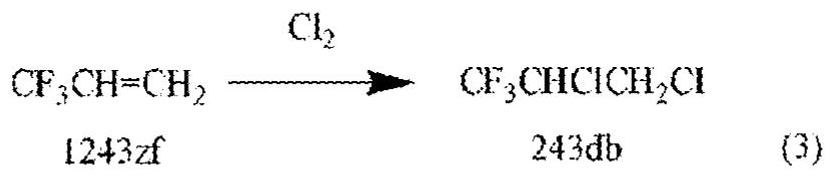
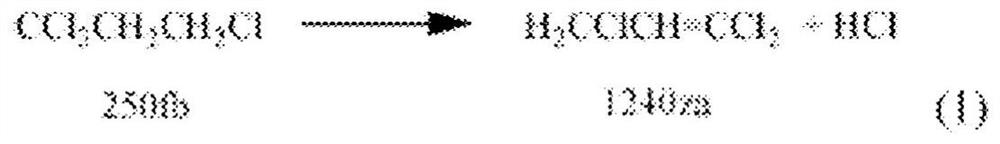Compositions and methods for an integrated 2,3,3,3-tetrafluoropropene manufacturing process
A technology of tetrafluoropropene and composition, which is applied in the field of compositions for synthesizing hydrofluoroolefins, and can solve problems such as reduced selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] To a Hastelloy tube (12" by 1 / 2") was added 6cc of 10% Cr / AC catalyst (12 mesh-20 mesh). With 30 standard cubic centimeters per minute (sccm) of nitrogen (N 2 ) purged the catalyst bed and set the temperature to 150°C for 2 hours and 250°C for 2 hours. Lower the temperature to 200°C and the N 2 The flow was changed to 60 seem and anhydrous HF stream was fed at 20 seem for 1 hour. The temperatures were then set at 250°C and 300°C for 1 hour each. At 300°C, the N 2 The flow and HF flow were changed to 20 seem and 30 seem, respectively, for one hour. The HF flow was then changed to 48 seem and the temperature was changed to 325°C for 2 hours. stop after N 2 flow and the HF flow was maintained at 48 seem for 1 hour to complete catalyst activation.
Embodiment 2
[0091] Conversion of 1,1,3-trichloro-1-propene (1240za) to 3,3,3-trifluoro-1-propene (1243zf).
[0092] 1240za was fed at about 0.2 cc / hr using a syringe pump and HF was fed at about 12.5 seem using a mass flow controller. Before entering the reactor, the organic and HF streams were mixed in a gasifier heated at 150°C. The reaction was carried out at 300°C and atmospheric pressure. Sample analysis was performed by injecting the product stream directly onto an Agilent 7890A GC equipped with a 5975C MS. The gas chromatography column used to analyze the product stream was derived from 20 meters x 1 / 8" column.
Embodiment 3
[0094] Conversion of 1,1,1,3-tetrachloropropane (250fb) to 3,3,3-trifluoro-1-propene (1243zf) (comparative)
[0095] 250fb was fed at about 0.2 cc / hr using a syringe pump and HF was fed at about 12.5 seem using a mass flow controller. Before entering the reactor, the organic and HF streams were mixed in a gasifier heated at 150°C. The reaction was carried out at 300°C and atmospheric pressure. Sample analysis was performed by injecting the product stream directly onto an Agilent 7890A GC equipped with a 5975C MS. The gas chromatography column used to analyze the product stream was derived from 20 meters x 1 / 8" column.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com