Preparation method of 1, 3-dichloro-1, 1-difluoropropane
A technology of difluoropropane and tetrachloropropane, which is applied in the field of fluorine-containing chemicals, can solve the problem of low selectivity of difluoropropane, and achieve the effects of high selectivity, easy availability of raw materials, and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 5.4g of niobium pentachloride, 35.6g of antimony trifluoride, 30ml of 1-chloroethane and 52g of 1,1,1,3-tetrachloropropane into a 300mL stainless steel belt stirred autoclave, and start stirring. After the temperature was raised to 40°C, the reaction was carried out for 8 hours. After the reaction, the sample was washed with water, filtered, and deacidified, and then analyzed by gas chromatography. The conversion rate of 1,1,1,3-tetrachloropropane was 99.6%. 1,3-Dichloro- The selectivity to 1,1-difluoropropane was 93.3%.
Embodiment 2~7
[0022] Examples 2-7 Prepare 1,3-dichloro-1,1-difluoropropane according to the same method as in Example 1, the difference is that the catalyst, solvent and , reaction temperature and reaction time, reaction result is as shown in table 1.
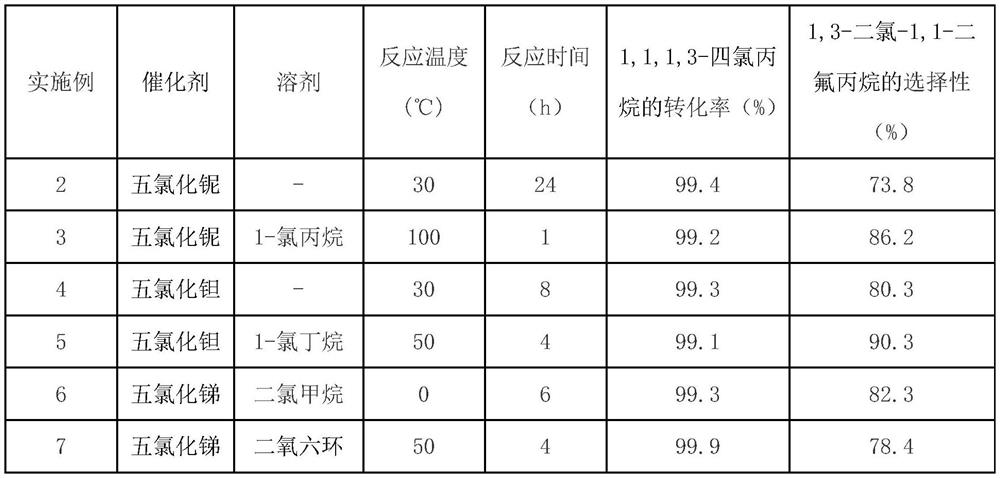
[0023] Table 1
[0024]
[0025] Table 1 shows that the polarity of the solvent can affect the solubility and acidity of the catalyst and substrate, which in turn affects the selectivity of the target product.
Embodiment 8~12
[0027] Examples 8-12 Prepare 1,3-dichloro-1,1-difluoropropane according to the same method as in Example 1, except that antimony trifluoride and 1,1,1,3-tetrachloropropane The molar ratio, the molar ratio of the catalyst to 1,1,1,3-tetrachloropropane, and the reaction results are shown in Table 2.
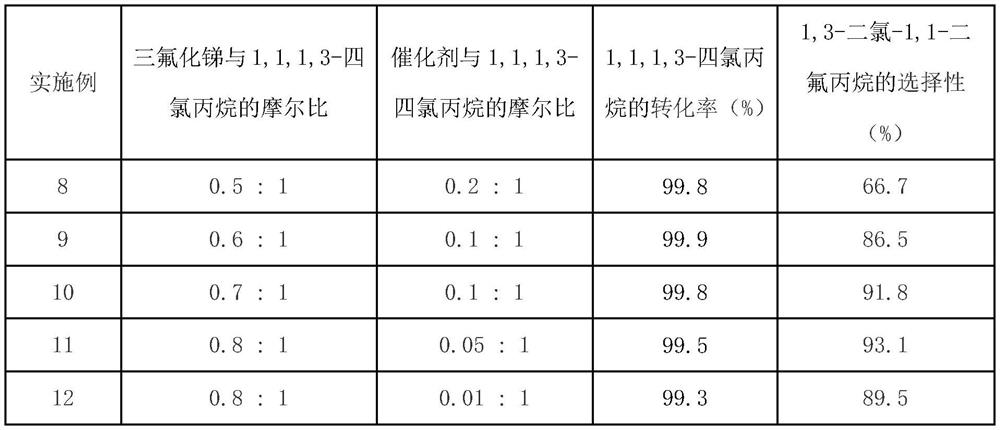
[0028] Table 2
[0029]
[0030] Table 2 shows that the conversion rate of raw material 1,1,1,3-tetrachloropropane is all above 99.0% within the range of above-mentioned experimental conditions, and can effectively improve target product 1,3-dichloropropane along with the increase of fluorination reagent consumption - The selectivity of 1,1-difluoropropane, when the molar ratio of the catalyst, antimony trifluoride and 1,1,1,3-tetrachloropropane is 0.05:0.8:1, the reaction selectivity is better, as 93.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com