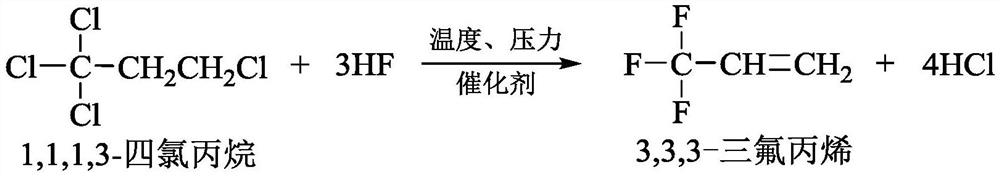
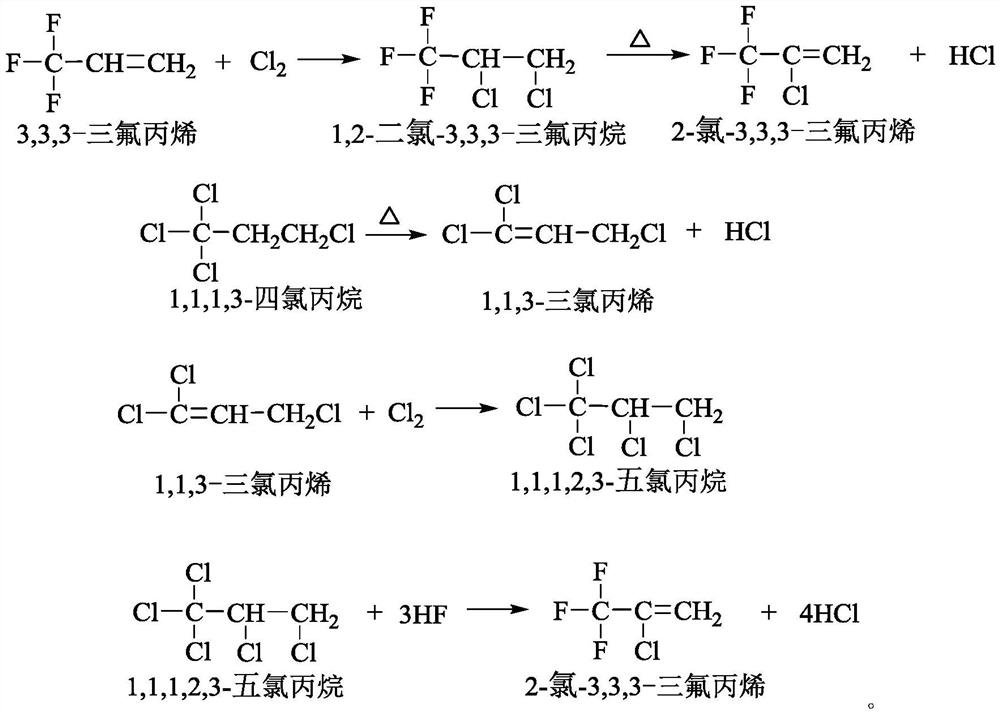
Co-production method and device for 3, 3, 3-trifluoropropene and 2-chloro-3, 3, 3-trifluoropropene
A technology of trifluoropropene and liquid chlorine, applied in chemical instruments and methods, organic chemistry, preparation of halogenated hydrocarbons, etc., can solve the problems of many reaction steps, slow reaction rate, low product quality, etc., and achieve the effect of efficient separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
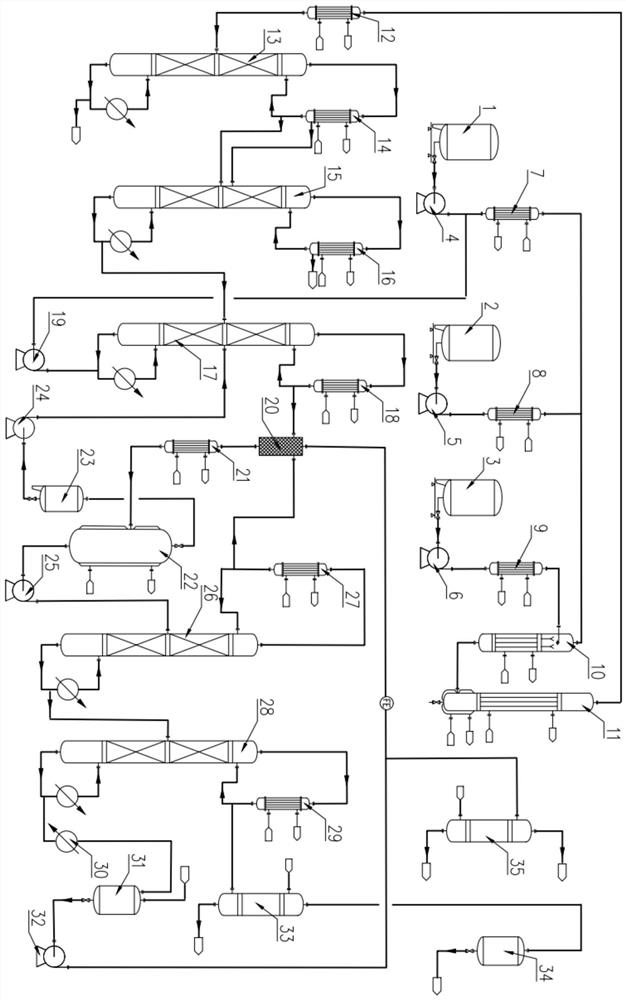
[0074] A co-production device for 3,3,3-trifluoropropene and 2-chloro-3,3,3-trifluoropropene, which includes a reaction system, a rectification pre-separation system, and an azeotrope-like extraction and separation system part;
[0075] The reaction system comprises hydrogen fluoride metering tank 1, liquid chlorine metering tank 2, tetrachloropropane metering tank 3, hydrogen fluoride metering pump 4, liquid chlorine metering pump 5, tetrachloropropane metering pump 6, hydrogen fluoride vaporizer 7, liquid chlorine vaporizer 8 , tetrachloropropane preheater 9, premixer 10, reactor 11, reaction cooler 12, described hydrogen fluoride metering tank 1 is communicated with hydrogen fluoride vaporizer 7 bottom feeding ports through hydrogen fluoride metering pump 4, hydrogen fluoride vaporizer 7 top outlets The feed port is connected to the gas phase feed port at the top of the premixer 10, the liquid chlorine metering tank 2 is connected to the feed port at the bottom of the liqui...
Embodiment 2
[0080] A co-production device for 3,3,3-trifluoropropene and 2-chloro-3,3,3-trifluoropropene as described in Example 1, the difference is: the fixed bed reactor with tubes 11. The lower head is provided with a jacket, and heat transfer oil is introduced into the jacket for heating to ensure that the feed temperature is further increased to the reaction temperature. The rectification II tower condenser 16 is cooled by -30°C to -35°C frozen brine, Further strengthen the cooling effect of other materials except hydrogen chloride, reduce material loss, and increase yield. The jacket of the phase separation condenser 21 is cooled by frozen brine with a temperature of -30°C to -35°C. The phase separator 20 The jacket is cooled by frozen brine with a temperature of -30°C to -35°C, and the upper part of the 3,3,3-trifluoropropene alkali washing tower 33 is connected with an alkali liquid input pipeline, and the 2-chloro- The middle and lower part of the 3,3,3-trifluoropropene alkali w...
Embodiment 3
[0082] Utilize the method for the coproduction of 3,3,3-trifluoropropene and 2-chloro-3,3,3-trifluoropropene by the production device described in embodiment 1 or 2, the fluorination catalyst used in the embodiment is chromium series Fluorination catalyst, comprising steps as follows:
[0083] (1) Reaction process
[0084] a) Hydrogen fluoride is pumped to hydrogen fluoride vaporizer 7 by hydrogen fluoride metering tank 1 to preheat, b) Liquid chlorine is pumped to liquid chlorine vaporizer 8 by liquid chlorine metering tank 2 to preheat, c) 1,1 is pumped by tetrachloropropane metering tank 3 , 1,3-tetrachloropropane is pumped to the tetrachloropropane preheater 9 for preheating, and the materials preheated in steps a), b), and c) are simultaneously fed into the falling film evaporation premixer 10 continuously, and the falling film evaporation premixer 10 is The temperature inside the mixer 10 is 240-300°C, the working pressure is 1.0-1.3MPa, the molar ratio of chlorine gas ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| global warming potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com