Process for the production of chlorinated propanes
A technology for tetrachloropropane and pentachloropropane, applied in the field of producing chlorinated propane, can solve the problems of suboptimal commercial application, difficult processing conditions of pentachloropropane, etc., and achieve the effects of cost saving, time saving, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
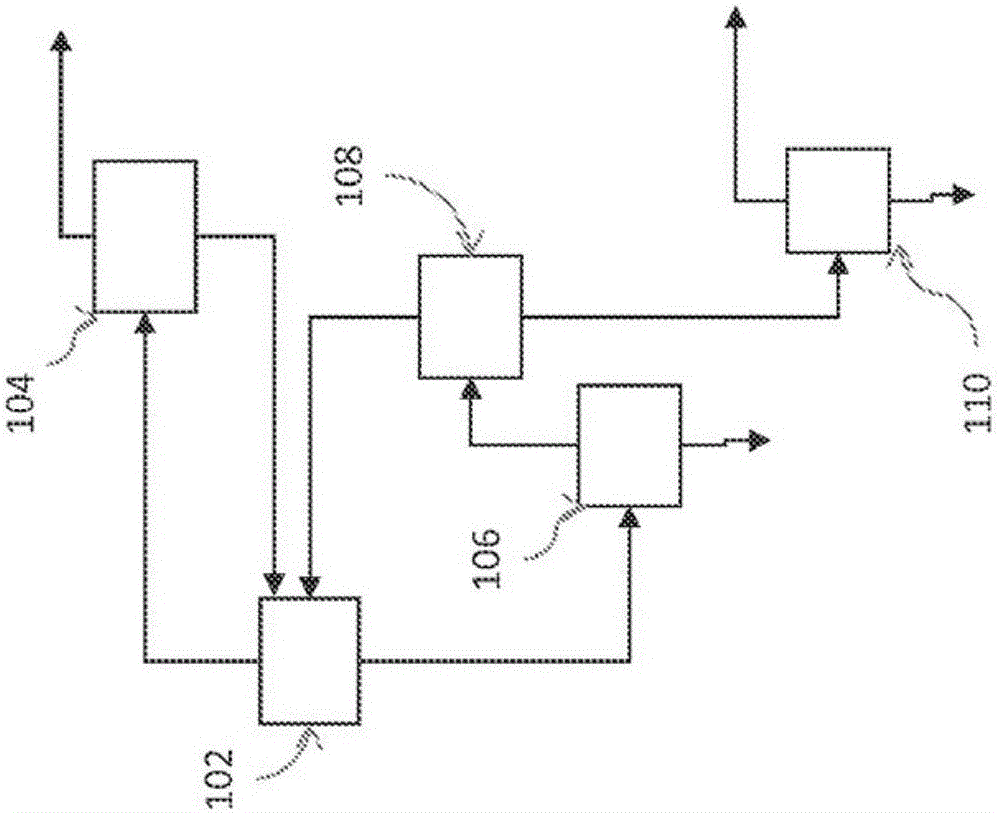
Image
Examples
example I
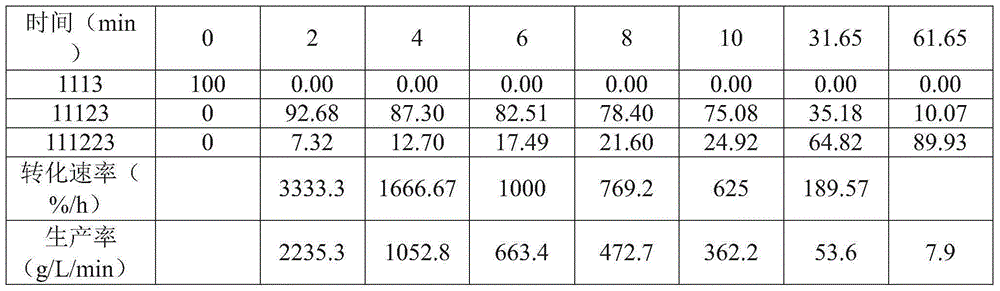
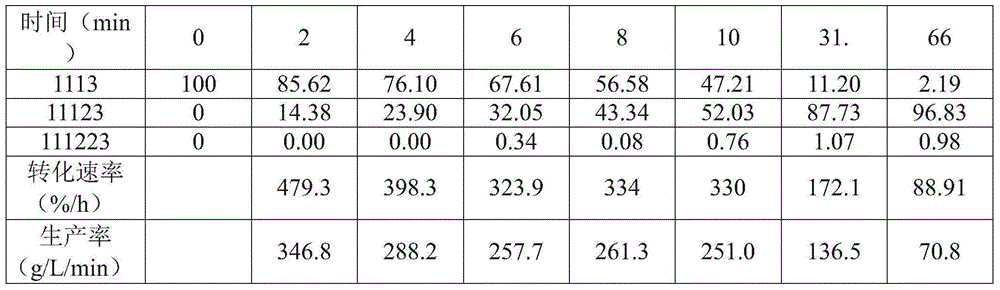
[0043] Example I: Using AlCl 3 to FeCl 3 Chlorination of 1,1,1,3-tetrachloropropane
[0044] In the glove box, 100mg FeCl 3 or AlCl 3 and dichloromethane (45 mL) were charged to the bottom of a 100 mL Parr reactor. Seal the reactor, start stirring (900 rev / min), use N 2 (~140 psig) The reactor was pressurized and vented. Make chlorine gas (30% in N 2 Middle) was passed through the reactor for 35 minutes at a reactor pressure of 125 psig. 1,1,1,3-Tetrachloropropane (1 mL) and dichloromethane (9 mL) were charged to the addition pot. The chlorine gas was then stopped, the reactor was heated to 50°C and the reactor pressure was adjusted to ~125 psig. Addition tanks were added and the reactor was sampled every two minutes for 10 minutes, followed by samples at 30 and 60 minutes. The sample was removed from the box and quenched with saturated aqueous sodium bicarbonate. Separate the organic layer. by deuterated chloroform 1 H NMR spectral analysis indicated that for AlCl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com