A kind of preparation method of 1,1,1,3-tetrachloropropane
A technology of tetrachloropropane and carbon tetrachloride, which is applied in the preparation of halogenated hydrocarbons, chemical instruments and methods, catalysts for physical/chemical processes, etc. and other problems, to achieve the effect of improving the telomerization reaction rate, improving the telomerization reaction activity, and avoiding adhesion and agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
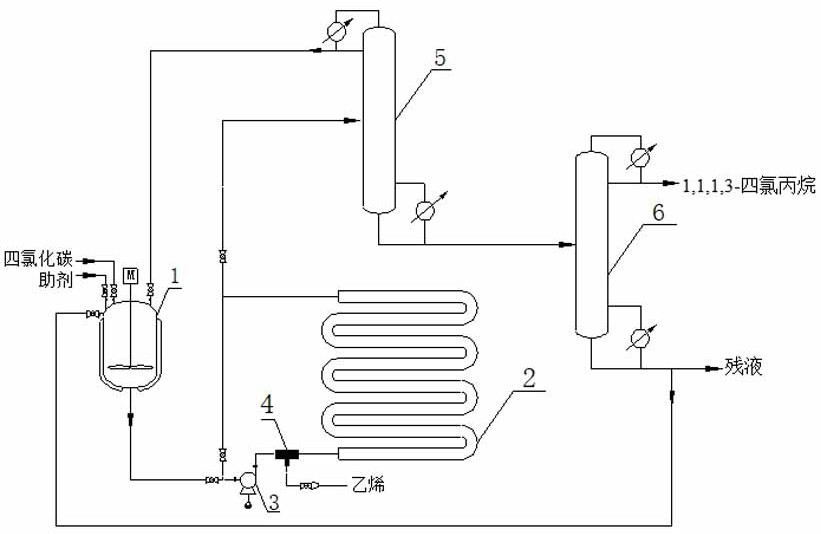
[0030] The reactor 2 uses an inner diameter of φ50mm, 32.0m long 32.0m, and a porous iron filler bed accumulated in an inner filled iron θ ring; 1,1,1,3-tetrachloropropane preparation Process is:
[0031] 1) After mixing the auxiliary (ethanol) and carbon tetrachloride, it is preheated to 74.6 ° C in the preheating kettle 1, and the mixture is added to the reinforcing reactor 2, and the circulating pump 3 is used continuously The upper material of the reactor, and is opened to the bottom of the reactor 2, so that the mixture is formed to mix uniform and cycle 6h;
[0032] 2) Relium the reactor temperature rise to 89 ° C, the gas-liquid mixer 4 is provided with a venturi mixer, ethylene feed amount feed amount feeds with ethylene and tetrachlormosphere ratio 1.09: 1.0, in the venturi mixer After mixing the liquid material of the pump cycle, the reinforcing reactor 2 is added, and the reactor pressure is 1.49 MPa. During the reaction, iron θ ring fillers and small amount of water in...
Embodiment 2
[0036] The difference from the first embodiment is that the ethylene, tetrachloride and the auxiliary fraction obtained in the top of the column is obtained in step 3) and circulate to step (1), the second rectification tower 6 tower kettle 75 ~ 95% residue cycle to step (2) re-use, the above-mentioned circulation reaction was once finally previously obtained 1,1,1,3-tetrachloropropane to obtain 1,1,1,3-tetrachloropropane, yield of 78%. The purity is 99.5%.
Embodiment 3
[0038] The difference from the first embodiment is that the reaction does not add auxiliaries, and the sample analysis is obtained in step 2), and the carbon tetrachloride is found to be substantially non-conversion.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com