One-pot method for preparing 1,1,1,2,3-pentachloropropane with high selectivity and high yield
A pentachloropropane and tetrachloropropane technology, applied in the field of compound synthesis, can solve the problems of coking and blackening of the reaction solution, catalyst deactivation, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
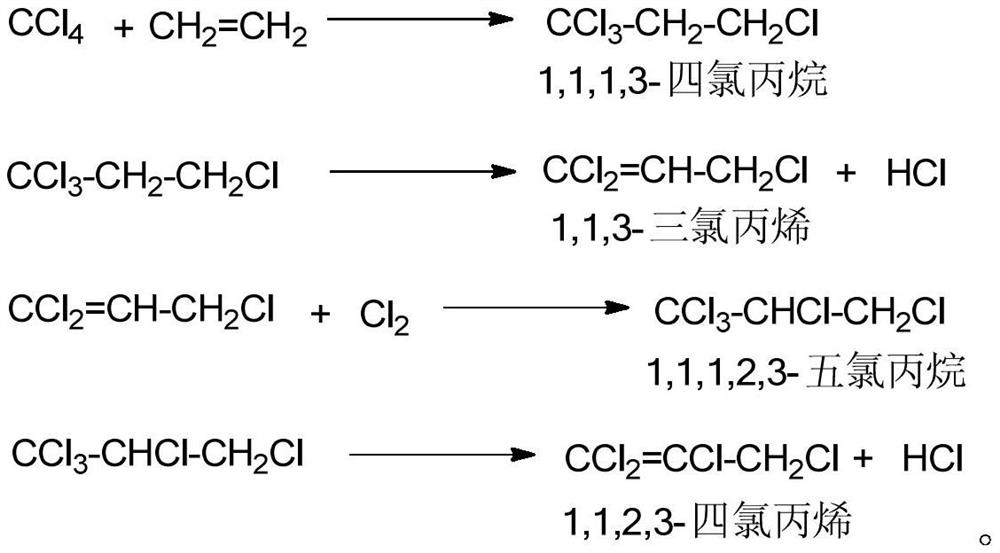
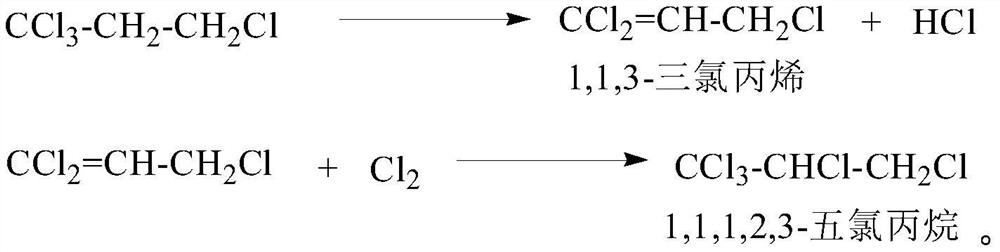
[0037] In a 500mL three-necked flask, add 300g of 1,1,1,3-tetrachloropropane with a content of 98% (the total content of water, alcohol, amine and trialkyl phosphate is 0.2%, and the content of carbon tetrachloride is 1.70%) ), 0.45g anhydrous ferric trichloride, put into the water bath of 75 ℃, stirred and reacted for 40 minutes, at this moment, the content of detecting 1,1,3-trichloropropene was 16%, and the reaction solution became light yellow, and the The temperature of the reaction system is reduced to 55°C, and chlorine gas is introduced, and the temperature of the reaction system is slowly raised to 70°C, and the temperature of the reaction system is controlled to be stable at 72°C. Gas chromatography detects 1,1,3-trichloropropene (area normalization method), so that the content of 1,1,3-trichloropropene is kept at 3%, and a small amount of chlorine gas and hydrogen chloride gas produced are absorbed with liquid caustic soda After reacting with chlorine gas for four h...
Embodiment 2
[0039] In a 500mL three-necked flask, add 300g of 98% 1,1,1,3-tetrachloropropane (the total content of water, alcohol, amine and trialkyl phosphate is 0.05%, and the carbon tetrachloride content is 1.77%) ), 0.45g anhydrous ferric chloride, 0.15g iron powder, put into 80 ℃ water bath, stir and react for 30 minutes. It is light yellow, reduce the temperature of the reaction system to 50°C, start to feed chlorine gas, slowly raise the temperature of the reaction system to 65°C, control the temperature of the reaction system to be stable at 75°C, and pass the chlorine gas at a rate of , reaction solution sampling detects 1,1,3-trichloropropene (area normalization method) with gas chromatography so that 1,1,3-trichloropropene content remains on 6%, a small amount of chlorine that emerges and the hydrogen chloride gas that produces Absorbed with liquid alkali, reacted with chlorine gas for 3 hours, took the reaction solution and detected it by gas chromatography, there were unreact...
Embodiment 3
[0041] In a 500mL three-necked flask, add 300g of 98% 1,1,1,3-tetrachloropropane (the total content of water, alcohol, amine and trialkyl phosphate is 0.14%, and the carbon tetrachloride content is 1.73%) ), 3g anhydrous ferric trichloride, put into 70 DEG C of water baths, stir and react for 20 minutes, at this moment, the content of detecting 1,1,3-trichloropropene is 12%, and the reaction solution becomes pale yellow, and the reaction The temperature of the system was lowered to 65°C, and chlorine gas was introduced. The temperature of the reaction system was slowly raised to 75°C. The temperature of the reaction system was controlled to be stable at 75°C. Chromatography detects 1,1,3-trichloropropene (area normalization method,) to keep its content at 1%, a small amount of chlorine gas that emerges and the hydrogen chloride gas produced are absorbed with liquid caustic soda, and after 3.5 hours of chlorine gas reaction, take The reaction solution was detected by gas chroma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com