Green method for preparing pinacolone
A green and formaldehyde-based technology, applied in the preparation of heterocyclic compounds, sustainable manufacturing/processing, chemical industry, etc., can solve the problems of increasing environmental protection costs and environmental pollution, uneconomical use of hydrochloric acid, large equipment investment, etc., to achieve equipment investment and the reduction of the site area, the obvious reduction of environmental protection costs and investment, and the effect of saving the exhaust gas recovery device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
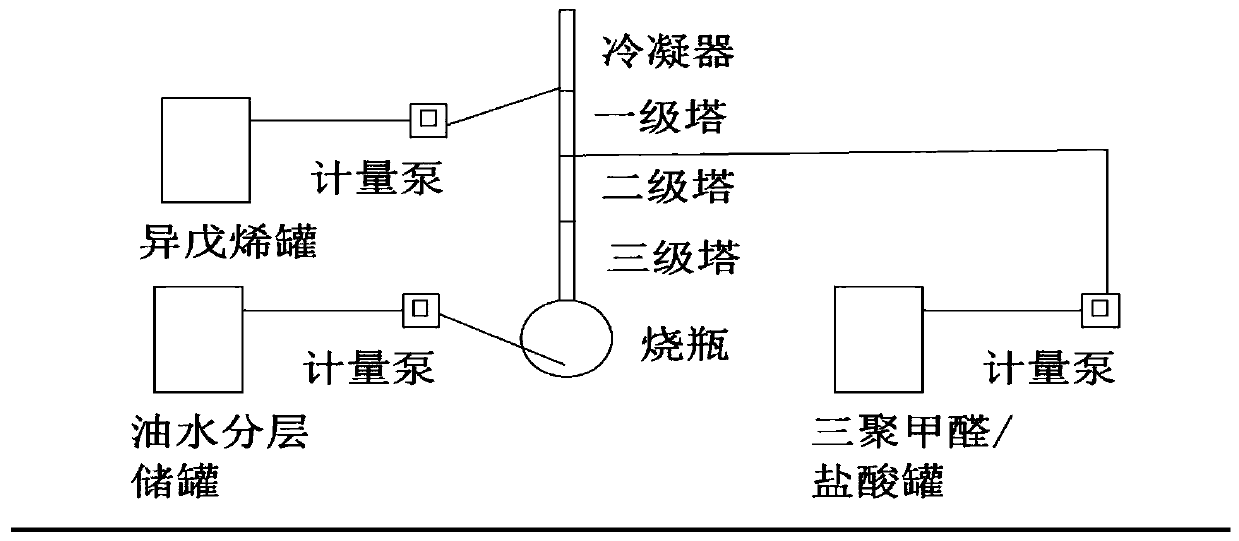
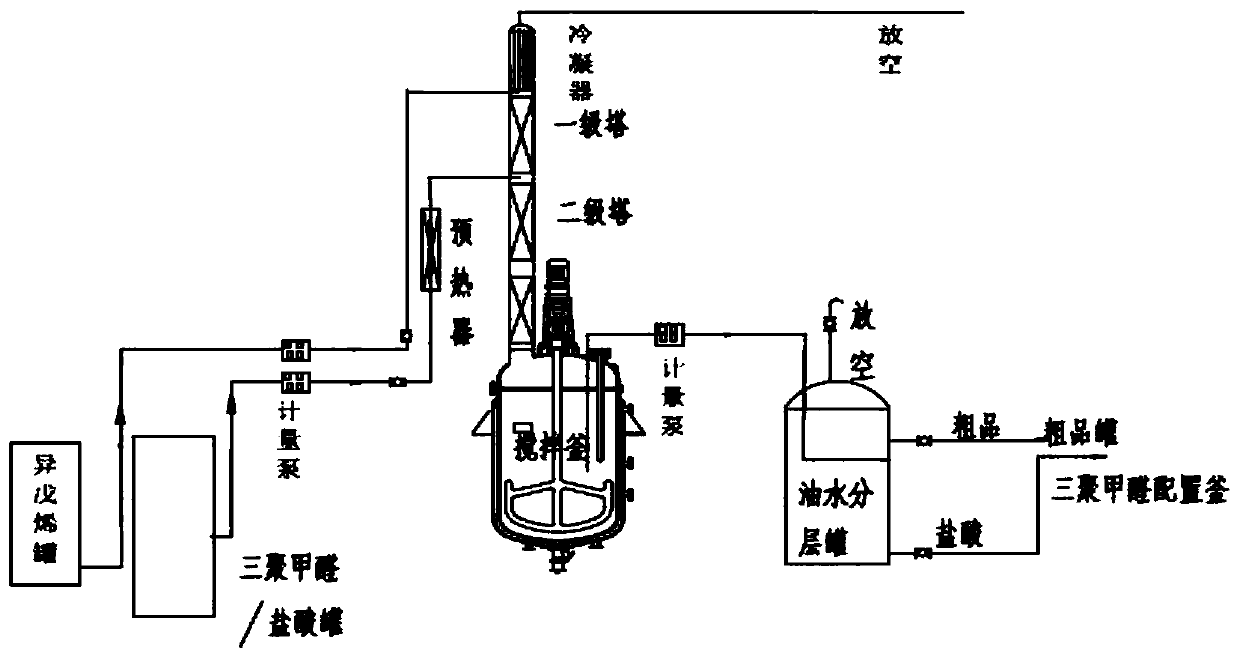
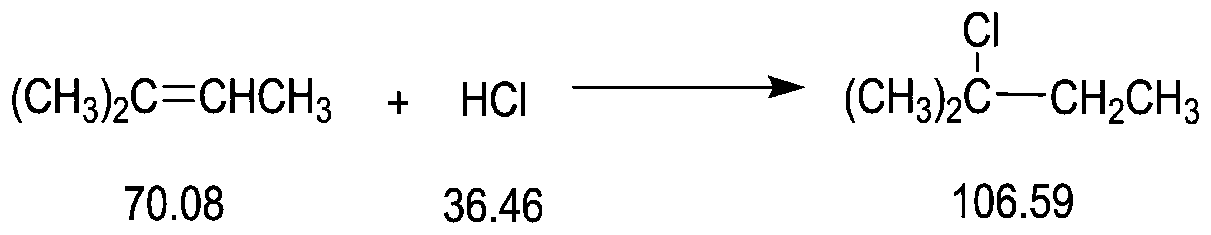
[0037] A 1L four-neck flask for the reaction was connected with a 1m packing column, the top of the packing column was connected to a condenser, a peristaltic pump was used for feeding and discharging, the cooling medium of the condenser was 20°C circulating water, and the four-necked flask was kept at 100°C. Before the reaction, 500 ml of reaction solution was added to the four-neck flask (reaction solution using 80 g of isoamylene raw material, the process was as in Comparative Example 1, and it was started as a full kettle) and the temperature was raised to 100°C. Isopentene (relative specific gravity 0.65, cooled to 10° C.) was fed at a speed of 2 g / min, and after 10 minutes, the paraformaldehyde solution was added with 255 grams of trioxane at a speed of 2.6 g / min (500 grams of 25% hydrochloric acid solution). POM is kept at 60°C, the relative specific gravity is 1.2) feeding, and the material is continuously discharged after 5 minutes. The water phase obtained by separati...
Embodiment 2
[0039] A 200L reactor is used for the reaction, connected to a packed tower with three sections of 2 meters and a diameter of 0.2 meters, and a square condenser is connected externally, and a metering pump is used for feeding and discharging. The condenser is fed with water at 20°C, and the temperature of the reactor is controlled at 100°C. Before the reaction, first add 100L of reaction solution (reaction solution with isoamyl content of 16KG, the process is the same as that of Comparative Example 1) in the reaction kettle and heat up to 100°C. After stabilizing for 1 hour, feed 400 g / min of isoamyl with a pump . After 20 minutes, feed paraformaldehyde / hydrochloric acid solution (300 kilograms of 25% hydrochloric acid, 153 kilograms of paraformaldehyde) at 520 g / min, and discharge continuously at the bottom of the reactor after 10 minutes. The aqueous phase obtained by separation at the discharge port was detected to have a hydrochloric acid concentration of 24.9%. After addi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com