Genomic imprinting subtype hemangioblastoma diagnosis kit
A technology of hemangioblastoma and diagnostic kit, applied in the field of gene imprinted subtype hemangioblastoma diagnostic kit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
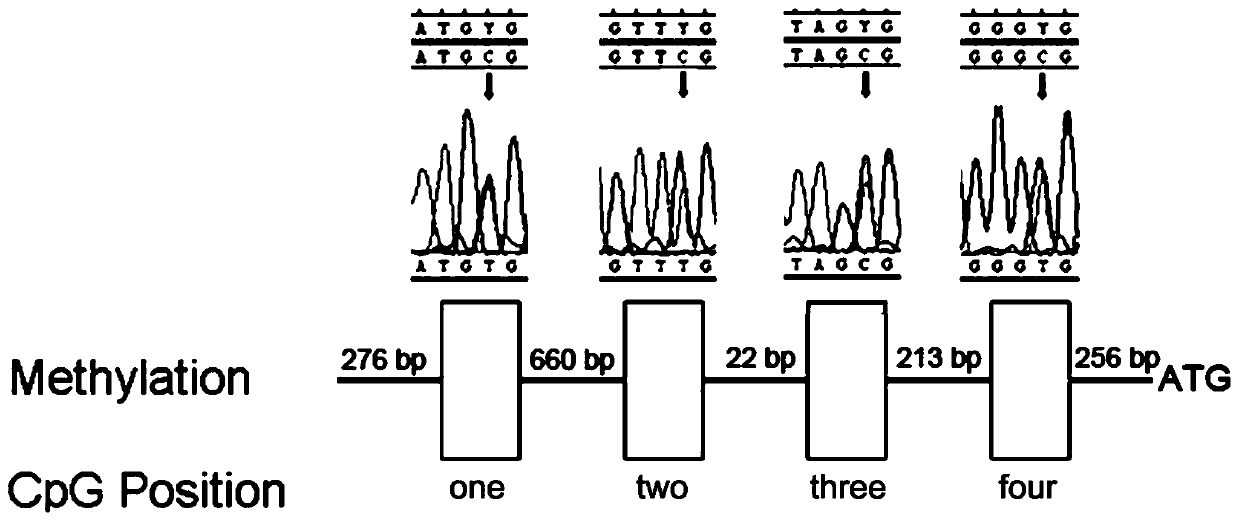
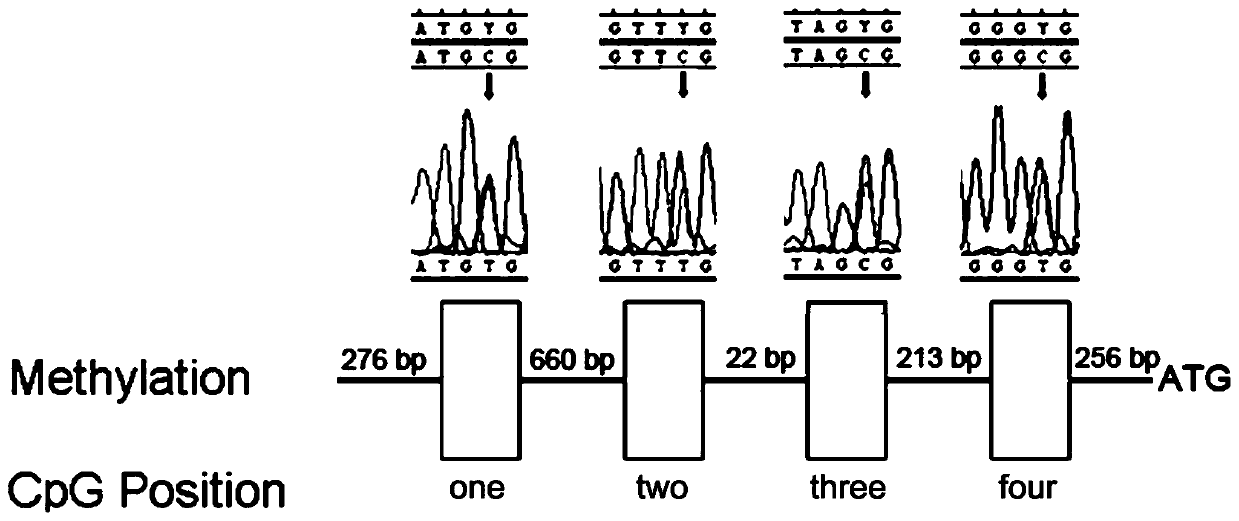
[0018] This embodiment provides a gene imprinted subtype hemangioblastoma diagnostic kit, which includes: reagents for bisulfite treatment of genomic DNA, reagents for VHL in methylation-specific PCR A primer set for amplification of the promoter, PCR buffer, and deoxynucleotide triphosphates and Taq DNA polymerase.
[0019] Wherein, the reagents for treating the genomic DNA with bisulfite include: 2M NaOH, 3M sodium bisulfite and 10 mM hydroquinone. These three reagents are contained in different containers respectively.
[0020] The primer set includes a first primer, a second primer, a third primer and a fourth primer.
[0021] The first primer includes a first upstream primer and a first downstream primer, wherein the sequence of the first upstream primer is shown in SEQ NO:1, and the sequence of the first downstream primer is shown in SEQ NO:2.
[0022] The second primer includes a second upstream primer and a second downstream primer, wherein the sequence of the second...
Embodiment 2
[0030] This embodiment provides a method for diagnosing gene imprinted subtype hemangioblastoma in human tissue or blood using the gene imprinted subtype hemangioblastoma diagnostic kit in Example 1.
[0031] The method includes the following steps:
[0032] Step 1. Genomic DNA extraction.
[0033] Genomic DNA was extracted from tissues or peripheral blood of subjects using QIAamp DNA mini Kit (Qiagen Inc, Chatsworth, CA), and then purified using Wizard Genomic DNA Purification Kit (Promega, Madison, WI, U.S.A.).
[0034] Step 2, bisulfite treatment.
[0035] Take 1 μg of purified genomic DNA, denature it with 2M NaOH, then incubate in 3M sodium bisulfite and 10 mM hydroquinone at 55°C for 17 hours, and then use the EpiTect Bisulfite kit (Axygen) to treat the genomic DNA with bisulfite Get modified DNA.
[0036] Step 3, methylation-specific PCR.
[0037] The PCR system includes: 1×PCR buffer (New England Biolabs), deoxynucleotide triphosphate (2.5mM each), primer set (0.8m...
Embodiment 3
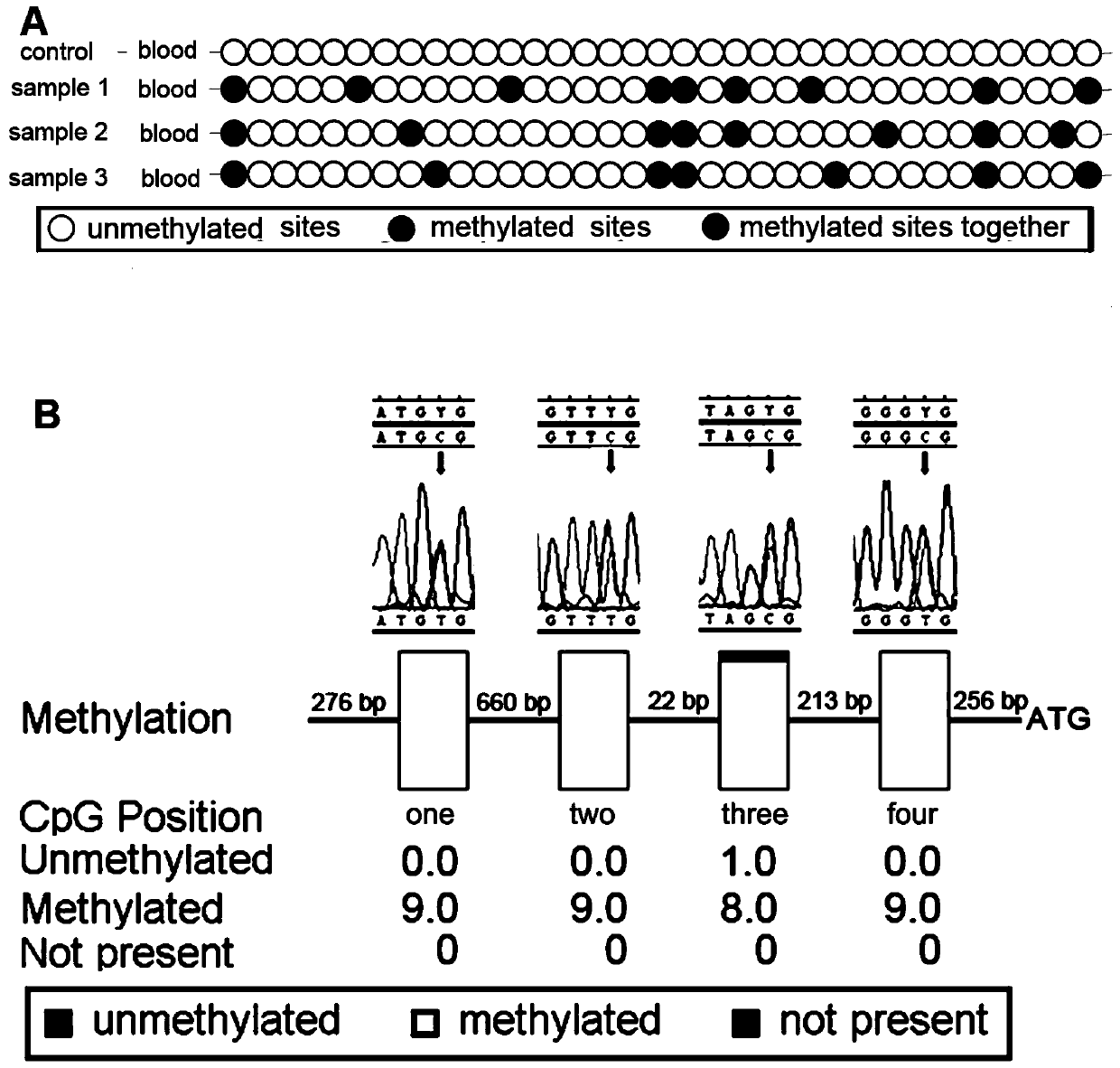
[0044] This example uses the gene imprinted subtype hemangioblastoma diagnostic kit in Example 1 and performs the gene imprinted subtype hemangioblastoma diagnostic test on the tissues or blood of three groups of subjects according to the diagnostic method in Example 2 .
[0045] Three groups of subjects include: experimental group, positive control group and negative control group.
[0046]Among them, the experimental group included three patients with confirmed hemangioblastoma due to the imprinted subtype, all of whom had no VHL gene mutation and all had hemangioblastoma. The positive control group included three hemangioblastoma patients with confirmed VHL gene mutations. The negative control group included three healthy volunteers.
[0047] In this embodiment, 5 mL of blood from three groups of subjects were collected as experimental samples for experiments.
[0048] The collection of samples from all the above subjects was approved by the Ethics Committee of Huashan H...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap