Neutralizing antibody against respiratory syncytial viruses and application thereof
A syncytial virus and anti-respiratory technology, applied in the fields of medicine and immunology, can solve problems such as severe toxicity and teratogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Screening, Expression and Purification of Anti-Respiratory Syncytial Virus Neutralizing Antibodies
[0059] 1. Preparation and identification of RSV Pre-F protein
[0060] According to the gene sequence of the pre-fusion conformation (Pre-F) protein of the RSV A2 strain fusion (F) protein in the NCBI database, the expression plasmid of RSV Pre-F protein was constructed with the pcDNA3.3 expression vector after adding the His tag, and transfected into 293T After the cells were cultured, the supernatant expressed by the cells was collected, concentrated and then purified by a nickel column to obtain the RSV Pre-F protein, which was then detected and identified.
[0061] 1) detection of expressed RSV Pre-F protein
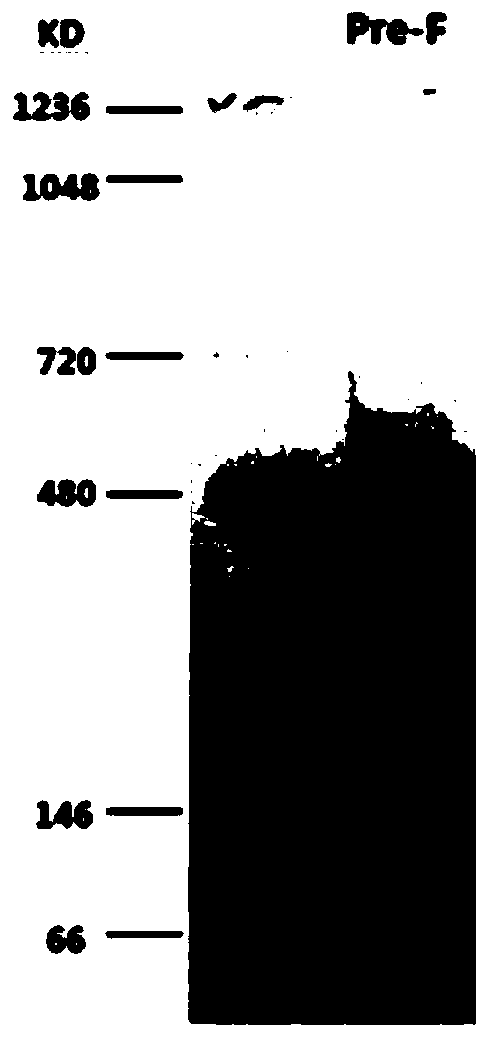
[0062] The expressed RSV Pre-F protein was detected by SDS-PAGE, and the result showed that its relative molecular weight was about 70kDa, which was consistent with the theoretical value.
[0063] The expressed RSV Pre-F protein was detected by Nati...
Embodiment 2
[0083] Example 2 Binding activity, in vitro micro-neutralizing activity and affinity experiments of neutralizing antibodies
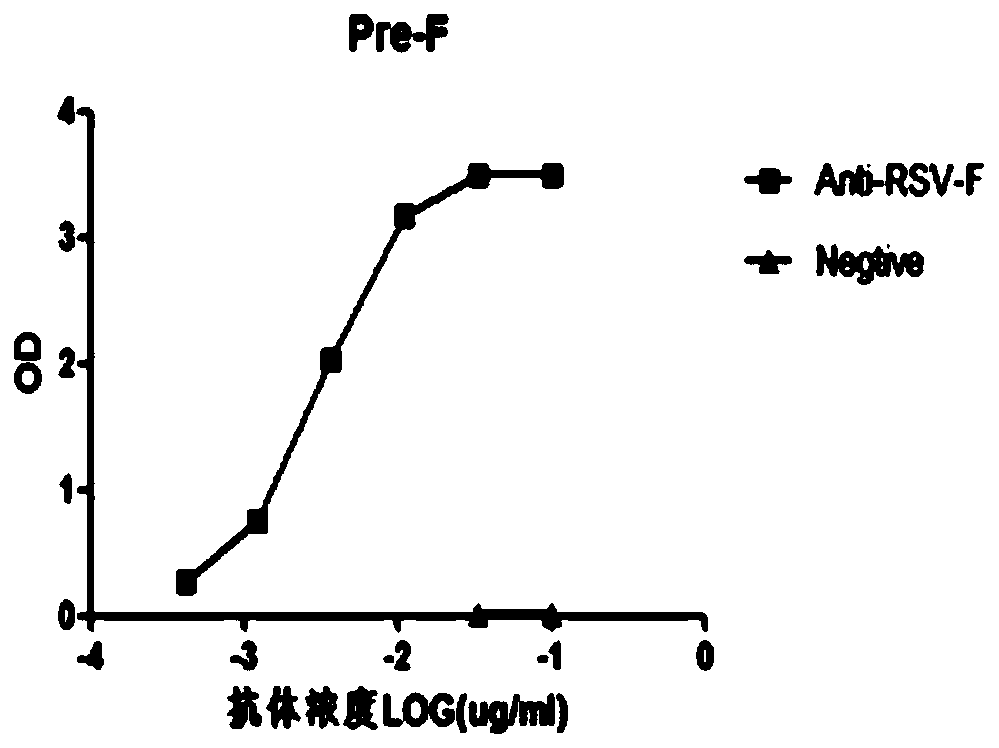
[0084] 1. Antibody binding activity
[0085] Use the same ELISA method mentioned above to detect the binding activity of the expressed and purified antibody:
[0086] The RSV Pre-F protein was coated with carbonate coating buffer, overnight at 4°C. Wash with PBST buffer, add blocking solution to block for 2h at 37°C or overnight at 4°C. Use the blocking solution to dilute the RSV antibody sample proportionally, starting at 1 μg / well, and diluting 12 gradients. Positive controls are positive plasma sample stock solution and RSV monoclonal antibody, 100 μL per well. Negative control was negative plasma sample stock solution and irrelevant antibody (TRN006) 0.5 μg / mL, 100 μL per well, blank plus 100 μL blocking solution, and incubated at 37°C for 1 hour. Wash with PBST buffer, add 100 μL of Goat-Anti-Human IgG-Fab-HRP and Goat-Anti-Mouse IgG-Fab-HRP di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com