Synthesis method of 2-amino-4H-pyran and derivative of 2-amino-4H-pyran
A synthesis method and derivative technology, applied in the field of synthesis of 2-amino-4H-pyran and its derivatives, can solve the problems of high cost, low yield, high reaction temperature, etc., and achieve the effect of increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
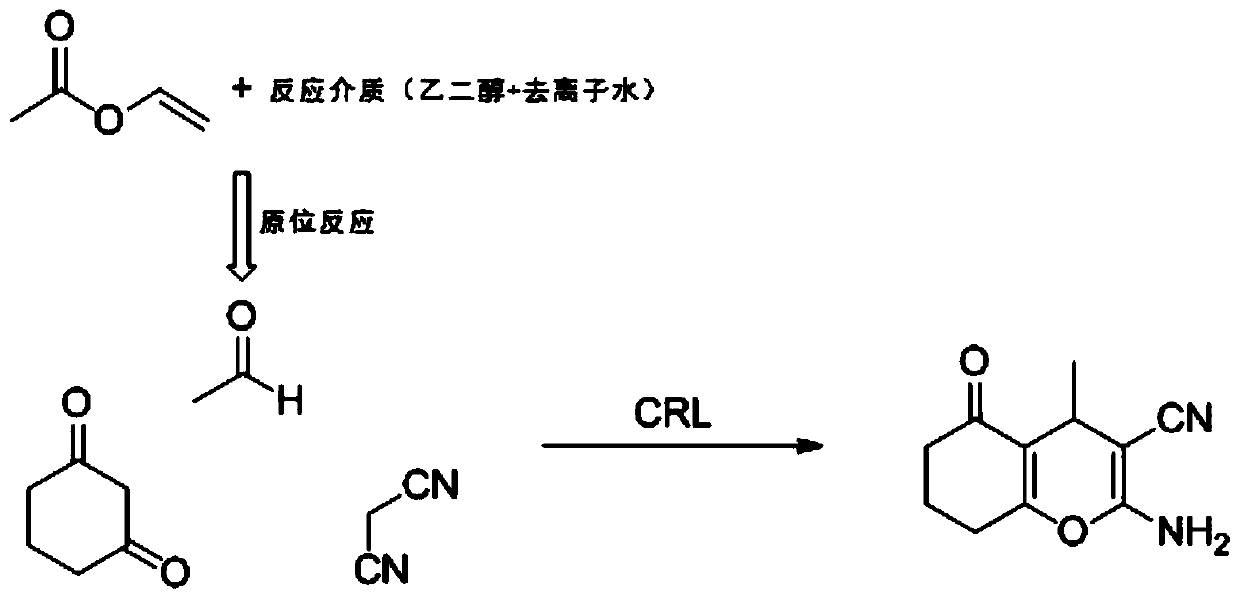
[0026] The synthetic method of 2-amino-4H-pyran of the present invention comprises: vinyl acetate, 1,3-dicarbonyl compound and malononitrile are mixed under the condition that reaction medium and catalyzer Candida rugosa lipase exist, in 2-Amino-4H-pyran and its derivatives were prepared by reacting in a constant temperature shaker at 30° C. with a rotational speed of 150 rpm for 30 minutes; wherein, the reaction medium included ethylene glycol and deionized water.
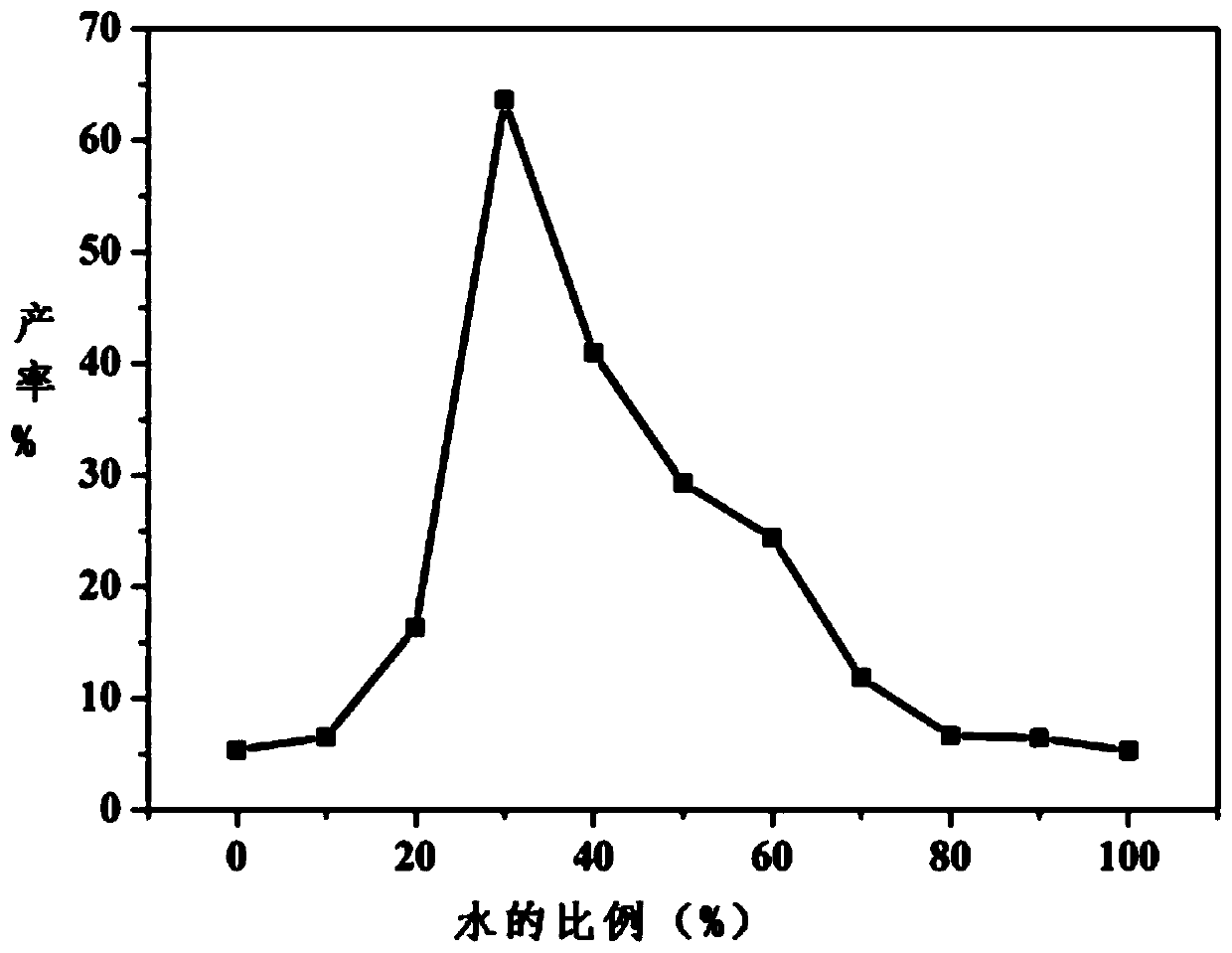
[0027] The proportioning relationship of vinyl acetate, 1,3-dicarbonyl compound, malononitrile, reaction medium and catalyst Candida rugosa lipase is: 0.2mL:0.05mmol:0.1mmol:10mL:2mg; wherein, in the reaction medium The volume ratio of ethylene glycol and deionized water is 6:4.
Embodiment 2
[0030] The 2-amino-4H-pyran method of the present invention comprises: vinyl acetate, 1,3-dicarbonyl compound and malononitrile are mixed under the condition that reaction medium and catalyzer Candida rugosa lipase exist, at 37 ℃ 1. React for 20 minutes in a constant temperature shaker with a rotating speed of 200 rpm to prepare 2-amino-4H-pyran and its derivatives; wherein, the reaction medium includes ethylene glycol and deionized water.
[0031] The proportioning relationship of vinyl acetate, 1,3-dicarbonyl compound, malononitrile, reaction medium and catalyst Candida rugosa lipase is: 0.4mL:0.1mmol:0.2mmol:10mL:5mg; wherein, in the reaction medium The volume ratio of ethylene glycol and deionized water is 7:3.
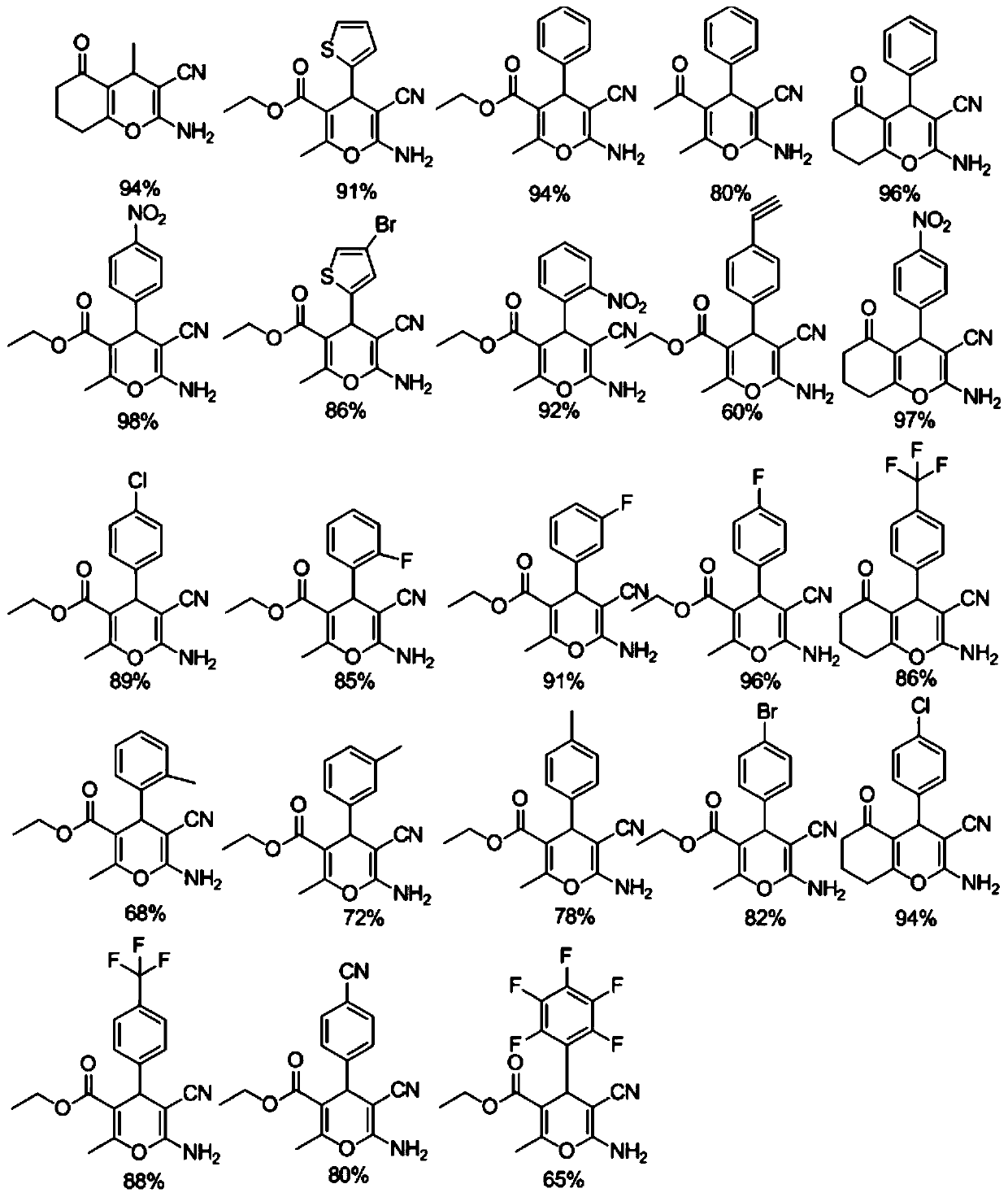
[0032] The yield of 2-amino-4H-pyran in this example is 96.8%.
Embodiment 3
[0034] The synthetic method of 2-amino-4H-pyran of the present invention comprises: vinyl acetate, 1,3-dicarbonyl compound and malononitrile are mixed under the condition that reaction medium and catalyzer Candida rugosa lipase exist, in 2-amino-4H-pyran and its derivatives were prepared by reacting in a constant temperature shaker at 50° C. and a rotation speed of 250 rpm for 15 minutes; wherein, the reaction medium included ethylene glycol and deionized water.
[0035] The proportioning relationship of vinyl acetate, 1,3-dicarbonyl compound, malononitrile, reaction medium and catalyst Candida rugosa lipase is: 0.6mL:0.2mmol:0.4mmol:10mL:10mg; wherein, in the reaction medium The volume ratio of ethylene glycol and deionized water is 8:2.
[0036] The yield of 2-amino-4H-pyran in this example is 95.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com