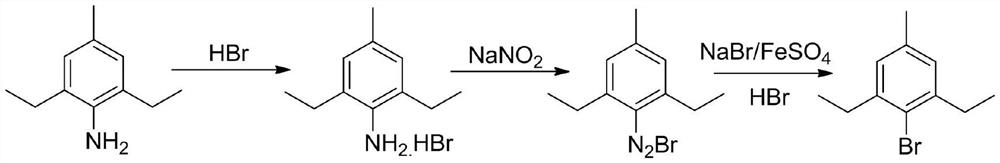
A kind of continuous flow preparation method of 2,6-diethyl-4-methyl bromobenzene
A technology of methyl bromide benzene and diethyl, which is applied in the field of preparation of pesticide intermediates, can solve the problems of increased content of by-products, cumbersome operation, and difficulty in controlling the content of by-products, and achieve the effect of high product yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
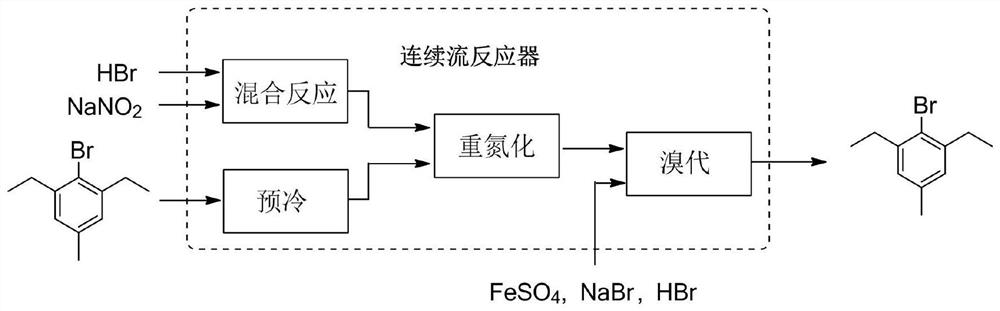
[0080] like figure 1 shown, taking 48wt% HBr aqueous solution and 25wt% NaNO 2 The aqueous solution was passed into the pre-cooling module at a flow rate of 37 g / min and 52 g / min, respectively, for mixing and pre-cooling at 5 °C, and the residence time was 7.3 s. Another 2,6-diethyl-4-methylaniline was taken into another pre-cooling module at a flow rate of 18 g / min and pre-cooled at 5 °C with a residence time of 26.6 s. The pressure of the microreactor was 0.6Mpa.
[0081] The pre-cooled material was passed into the next module for mixing and reacted at 5°C to form a diazonium salt intermediate with a residence time of 5.7s.
[0082] The generated diazonium salt intermediate continues to pass into the next bromination module, and at the same time according to 0.5eq FeSO 4 .7H 2 The solution was prepared in the ratio of O:2eq48%HBr:1eqNaBr and passed into the bromination reaction module at a speed of 64g / min, and the bromination reaction was completed at 80°C with a reside...
Embodiment 2
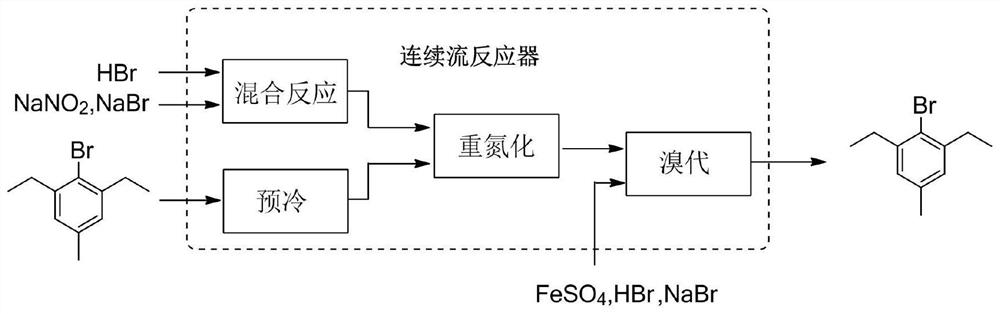
[0086] like figure 2 shown, take 48wt% HBr aqueous solution and 48wt% NaNO 2 / NaBr (1.7eq:2eq) aqueous solution was passed into the precooling module at a flow rate of 41.3g / min and 82.6g / min, respectively, for mixing and precooling at 5°C, and the residence time was 6.0s. Another 2,6-diethyl-4-methylaniline was taken into another pre-cooling module at a flow rate of 20 g / min and pre-cooled at 5 °C with a residence time of 24 s. The pressure of the microreactor was 0.9Mpa.
[0087] The pre-cooled material was passed into the next module for mixing and reacted at 5°C to form a diazonium salt intermediate with a residence time of 4.8s.
[0088] The generated diazonium salt intermediate continues to pass into the next bromination module, and at the same time according to 0.5eq FeSO 4 .7H 2 The bromination solution was prepared in the ratio of O:2eq48%HBr and passed into the bromination reaction module at a speed of 58.2g / min, and the bromination reaction was completed at 80°...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| fatigue bending times | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com