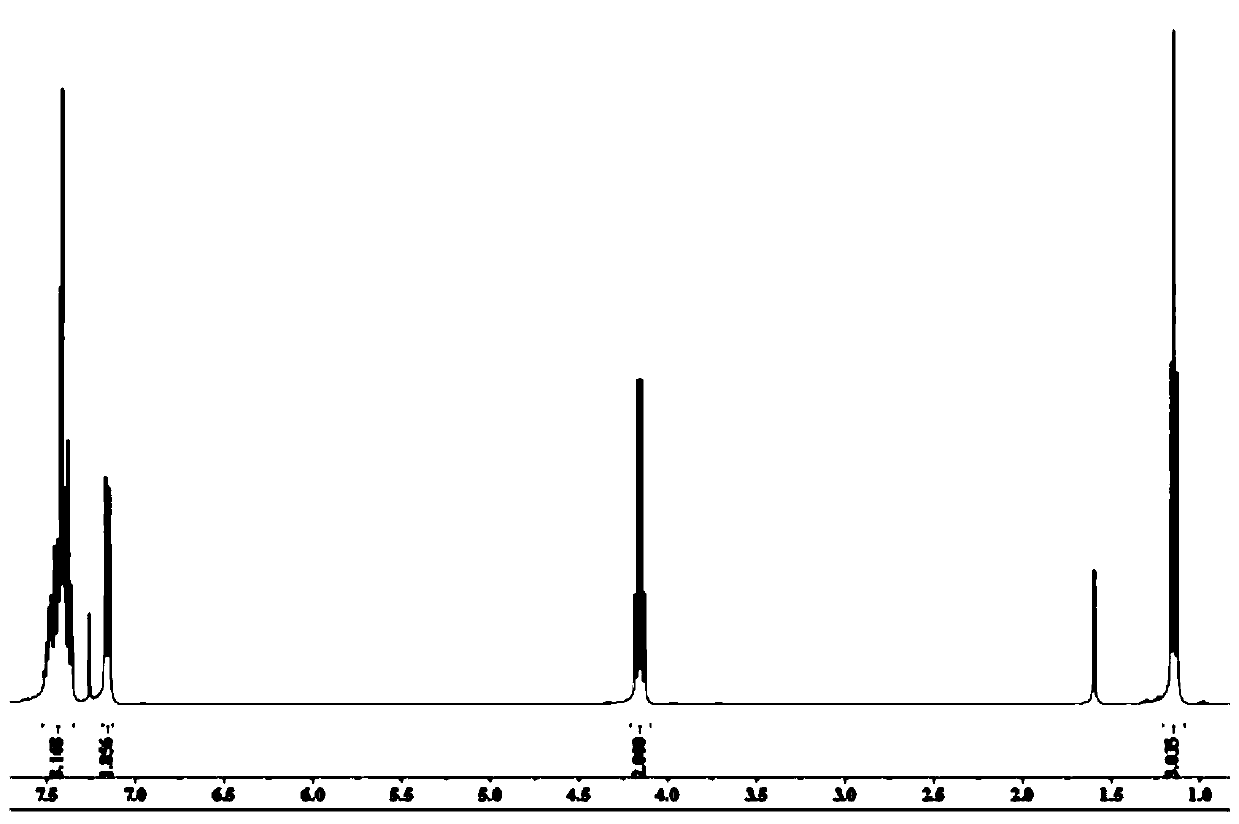
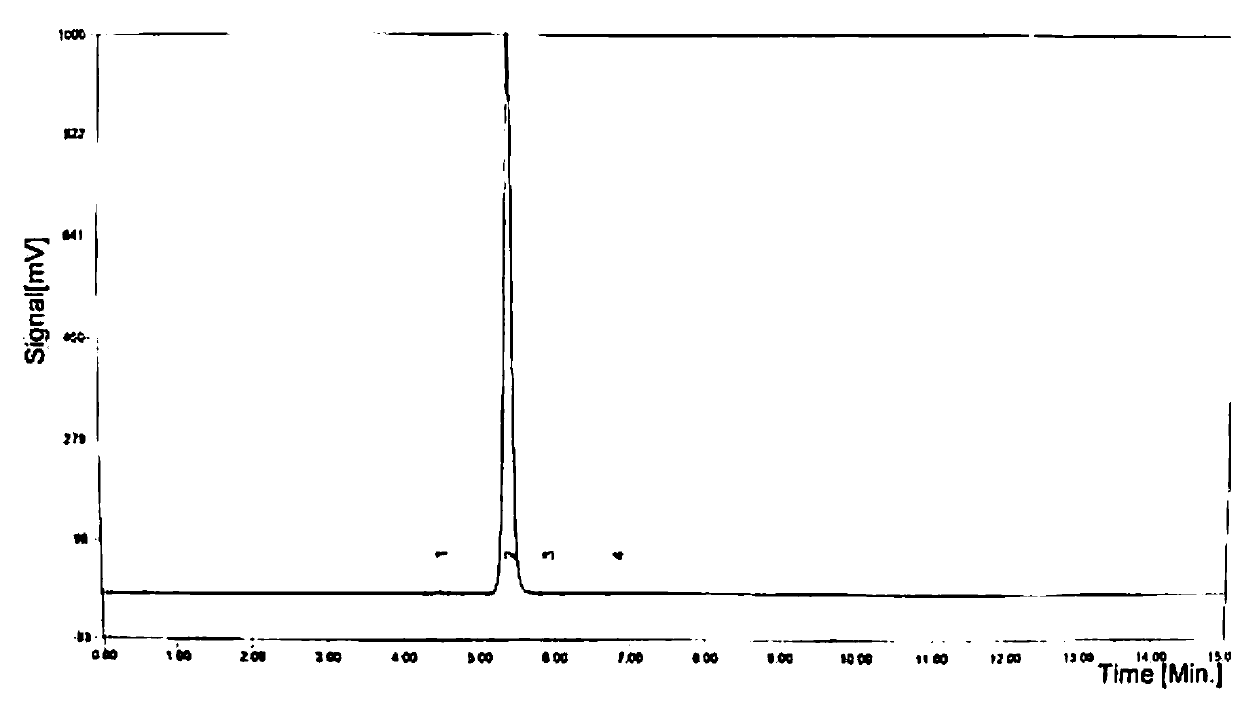
Preparation method of Etocrilene
A technology based on Lilin and the preparation process, applied in the preparation of organic compounds, carboxylic acid nitrile preparation, chemical instruments and methods, etc., can solve the problems of reduced reaction yield and achieve improved conversion rate, less impurities and high purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 (adding acetic acid midway)
[0031] Put 580kg of benzophenone, 520kg of ethyl cyanoacetate, 70kg of ammonium acetate and 60kg of acetic acid into the synthesis kettle in proportion, raise the temperature to 45°C, stir and dissolve; put on a vacuum, and gradually evacuate until there is an appropriate amount of distillate in the condenser (about -0.088Mpa), start to calculate the reaction time, continue to heat up to 72°C, keep warm, and gradually increase the vacuum until it reaches -0.095Mpa;
[0032] Add 50kg of preset amount of acetic acid to the reaction vessel, cancel the vacuum, and the reaction time is 10h;
[0033] After the reaction is over, turn on the circulating water to cool down to below 60°C under the condition of vacuum, add 300kg of water, stir for 20 minutes, stop the stirring, and keep it for 30 minutes. This process will cause stratification; extract the lower oil layer (the upper layer of waste water layer is discharged into the waste...
Embodiment 2
[0034] Embodiment 2 (adding acetic anhydride midway)
[0035] Put 580kg of benzophenone, 520kg of ethyl cyanoacetate, 70kg of ammonium acetate and 60kg of acetic acid into the synthesis kettle in proportion, raise the temperature to 45°C, stir and dissolve; put on a vacuum, and gradually evacuate until there is an appropriate amount of distillate in the condenser (about -0.088Mpa), start to calculate the reaction time, continue to heat up to 72°C, keep warm, and gradually increase the vacuum until it reaches -0.095Mpa;
[0036] Add 50kg of preset amount of acetic acid to the reaction vessel, cancel the vacuum, and the reaction time is 10h;
[0037] After the reaction is over, turn on the circulating water to cool down to below 60°C under the condition of vacuum, add 300kg of water, stir for 20 minutes, stop the stirring, and keep it for 30 minutes. This process will cause stratification; extract the lower oil layer (the upper layer of waste water layer is discharged into the ...
Embodiment 3
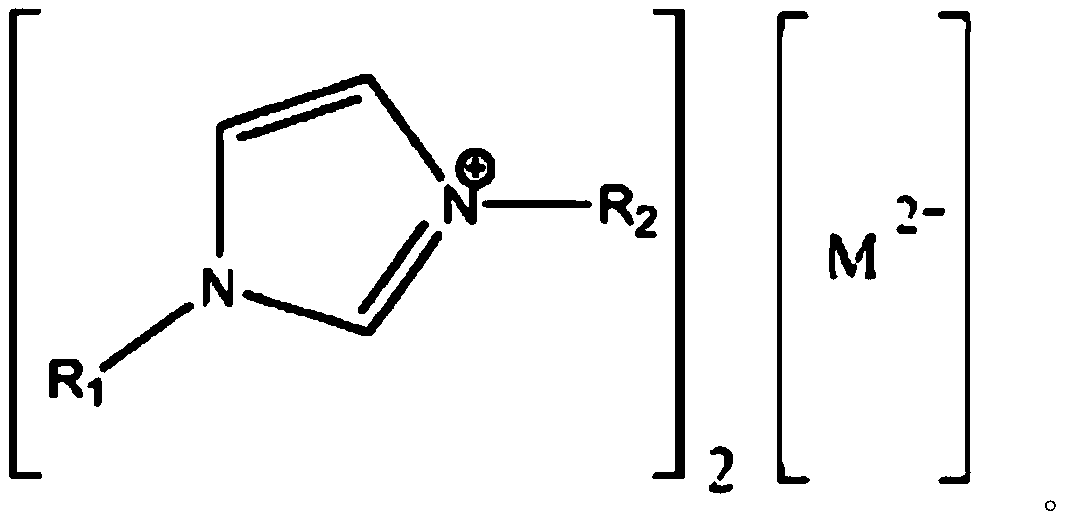
[0038] Embodiment 3 (addition of acetic anhydride+catalyst 1,3-dialkylimidazole oxometalate midway)
[0039] Put 580kg of benzophenone, 520kg of ethyl cyanoacetate, 70kg of ammonium acetate, 60kg of acetic acid, and 6.1kg of 1,3-dialkylimidazolium oxometalate into the synthesis kettle in proportion, raise the temperature to 45°C, and stir to dissolve; Apply vacuum and gradually evacuate until there is an appropriate amount of distillate in the condenser (about -0.088Mpa), start to calculate the reaction time, continue to heat up to 72°C, keep warm, and gradually increase the vacuum until it reaches -0.095Mpa;
[0040] Add 50kg of preset amount of acetic acid to the reaction vessel, cancel the vacuum, and the reaction time is 10h;
[0041] After the reaction is over, turn on the circulating water to cool down to below 60°C under the condition of vacuum, add 300kg of water, stir for 20 minutes, stop the stirring, and keep it for 30 minutes. This process will cause stratification...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com