Diclazuril derivative and application thereof and fungicide containing derivative
A derivative, clazuril technology, applied in the field of pesticides, can solve the problem of unclear action mechanism of diclazuril, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
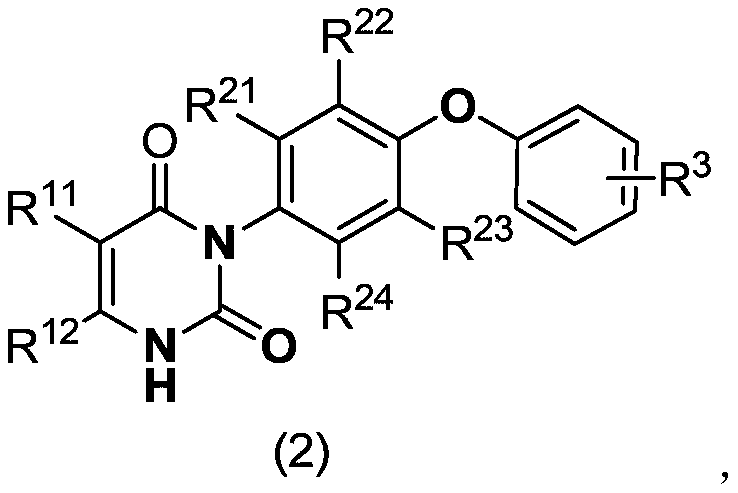
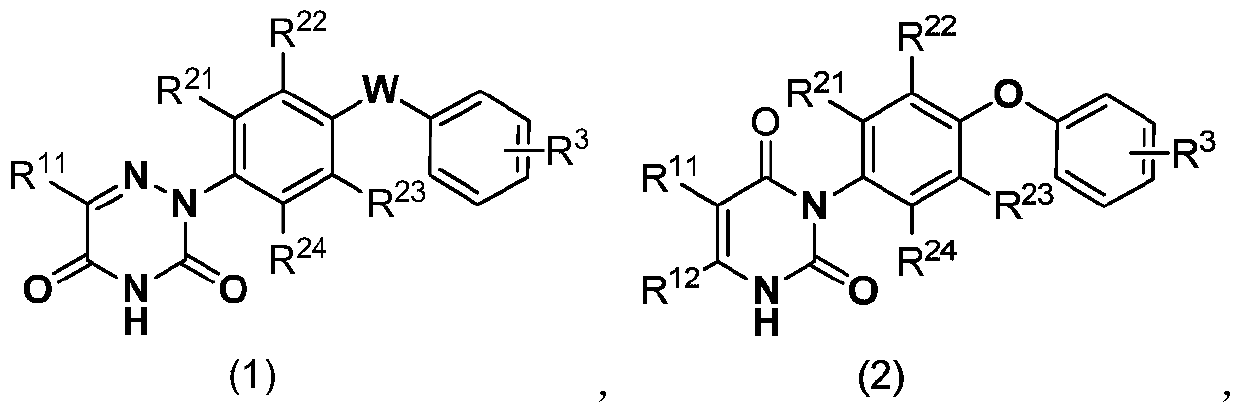
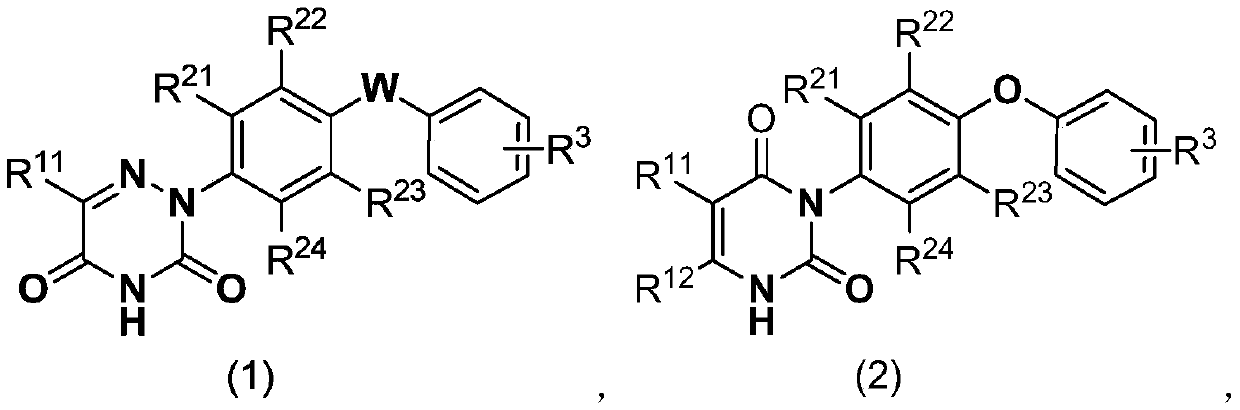
[0050] Specific embodiment 1: In formula (1) and formula (2),
[0051] R 11 And R 12 Respectively selected from H, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, methoxy, ethoxy, n-propoxy, isopropoxy, n Butoxy, isobutoxy, tert-butoxy, fluorine, chlorine, bromine, nitro, cyano, monobromomethyl, monochloromethyl, monofluoromethyl, trifluoromethyl and trifluoromethyl Fluoromethoxy;
[0052] R 21 , R 22 , R 23 And R 24 Respectively selected from H, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, methoxy, ethoxy, n-propoxy, isopropoxy, n Butoxy, isobutoxy, tert-butoxy, fluorine, chlorine and bromine;
[0053] R 3 Selected from H, C 1-4 The alkyl group, C 1-4 The halogenated alkyl group, C 1-4 的alkoxy, C 1-4 The halogenated alkoxy group, C 1-4 的alkylthio, C 1-4 At least one of halogenated alkylthio, nitro, cyano and halogen; and when R 11 , R 21 And R 24 For H, R 22 And R 23 When Cl and W is O; R 3 Not 4-Cl or 4-OCH 3 .
specific Embodiment approach 2
[0054] Embodiment 2: In formula (1) and formula (2),
[0055] R 11 And R 12 Respectively selected from H, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, methoxy, ethoxy, n-propoxy, isopropoxy, n Butoxy, isobutoxy, tert-butoxy, fluorine, chlorine, bromine, trifluoromethyl and trifluoromethoxy;
[0056] R 21 , R 22 , R 23 And R 24 Respectively selected from H, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl and chlorine;
[0057] R 3 Selected from H, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, methoxy, ethoxy, n-propoxy, isopropoxy, n-butyl Oxy, isobutoxy, tert-butoxy, methylthio, ethylthio, n-propylthio, isopropylthio, n-butylthio, isobutylthio, tert-butylthio, nitro, cyano At least one of radical and halogen; and
[0058] When R 11 , R 21 And R 24 For H, R 22 And R 23 When Cl and W is O; R 3 Not 4-Cl or 4-OCH 3 .
specific Embodiment approach 3
[0059] Specific embodiment 3: In formula (1) and formula (2),
[0060] R 11 And R 12 Respectively selected from H, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl and trifluoromethyl;
[0061] R 21 , R 22 , R 23 And R 24 Respectively selected from H, methyl, ethyl, n-propyl, isopropyl and chlorine;
[0062] R 3 Selected from H, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, methoxy, ethoxy, n-propoxy, isopropoxy, n-butyl Oxy, isobutoxy, tert-butoxy, methylthio, ethylthio, n-propylthio, isopropylthio, n-butylthio, isobutylthio, tert-butylthio, nitro, cyano At least one of radical and halogen; and
[0063] When R 11 , R 21 And R 24 For H, R 22 And R 23 When Cl and W is O; R 3 Not 4-Cl or 4-OCH 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com