Preparation method and application of specific CTL cell of target KRAS frequent mutation tumor
A KRAS-12D and KRAS-12V technology, applied in the field of tumor treatment, can solve the problems that patients with KRAS mutations are difficult to benefit, the treatment effect is not satisfactory, and the survival period is short
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The present invention provides a preparation method of specific CTL targeting KRAS multiple mutation tumors, comprising the following steps:
[0041] 1) mixing the mutant polypeptides KRAS-12D, KRAS-12C, KRAS-12V, KRAS-12A, KRAS-12S, KRAS-13D and KRAS-61H to obtain a KRAS mutant polypeptide mixture;
[0042] 2) adding DCs to the KRAS mutant polypeptide mixture described in step 1), and culturing to obtain antigen-presenting cells;
[0043] 3) Co-culture the antigen-presenting cells described in step 2) with peripheral blood mononuclear cells to obtain specific CTLs targeting KRAS multiple mutation tumors;
[0044] The amino acid sequences of KRAS-12D, KRAS-12C, KRAS-12V, KRAS-12A, KRAS-12S, KRAS-13D and KRAS-61H are shown in SEQ ID NO.1-7.
[0045] In the present invention, firstly, the mutant polypeptides KRAS-12D, KRAS-12C, KRAS-12V, KRAS-12A, KRAS-12S, KRAS-13D and KRAS-61H are mixed to obtain the KRAS mutant polypeptide mixture.
[0046] In the present invention, ...
Embodiment 1
[0062] 1. Select the KRAS mutant sequence:
[0063] KRAS-12D: TEYKLVVVGADGVGKSALTIQ
[0064] KRAS-12C: TEYKLVVVGACGVGKSALTIQ
[0065] KRAS-12V: TEYKLVVVGAVGVGKSALTIQ
[0066] KRAS-12A: TEYKLVVVGAAGVGKSALTIQ
[0067] KRAS-12S: TEYKLVVVGASGVGKSALTIQ
[0068] KRAS-13D: EYKLVVVGAGDVGKSALTIQL
[0069] KRAS-61H: CLLDILDTAGHEEYSAMRDQY
[0070] 2. Synthesis of KRAS mutant polypeptide:
[0071] The peptides are synthesized by GenScript Biotechnology Co., Ltd. The requirements for synthetic peptides: 98% purity, with FITC fluorescent label, each peptide is dissolved in 1640, the concentration is 1mg / mL, and it is ready for use.
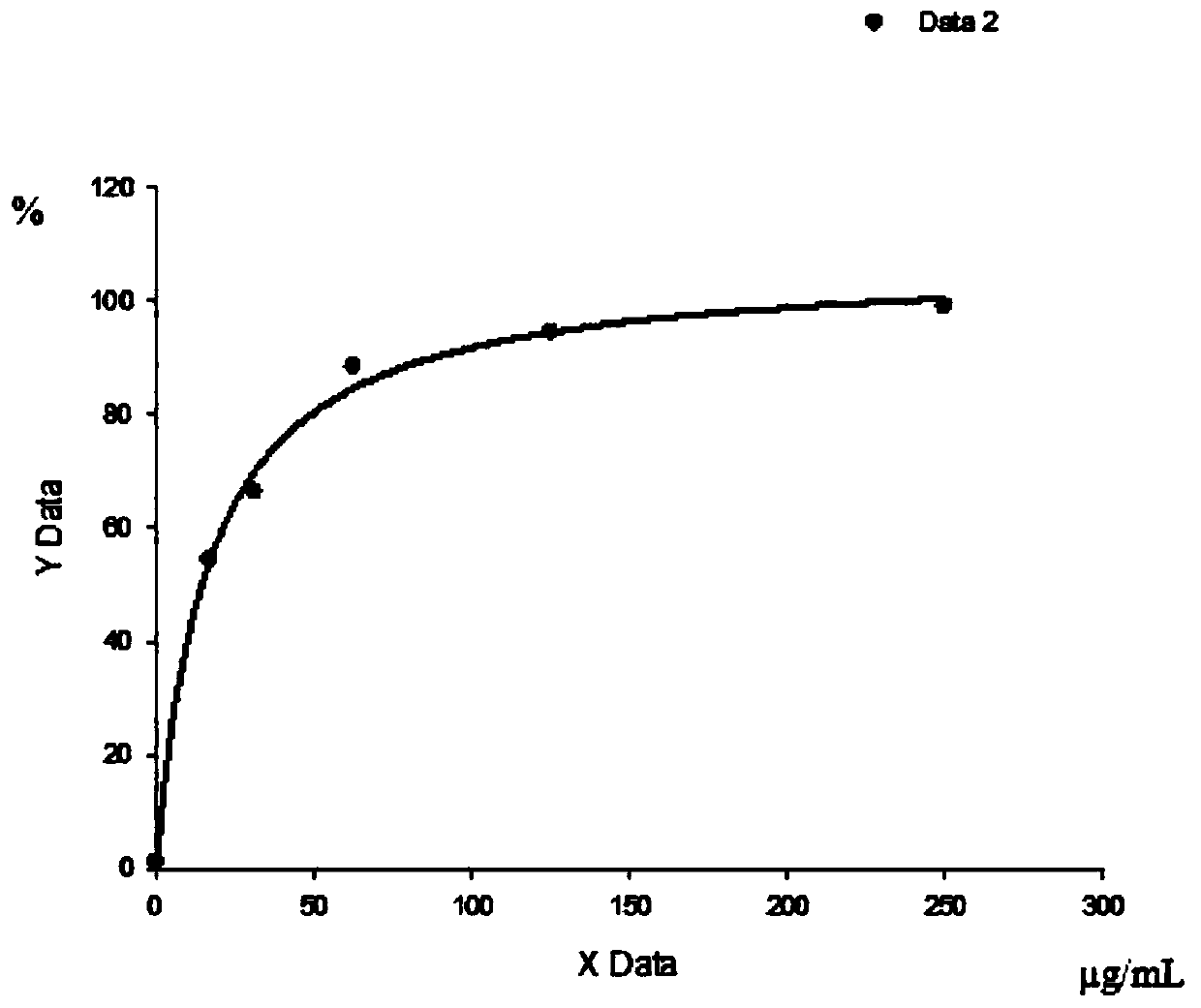
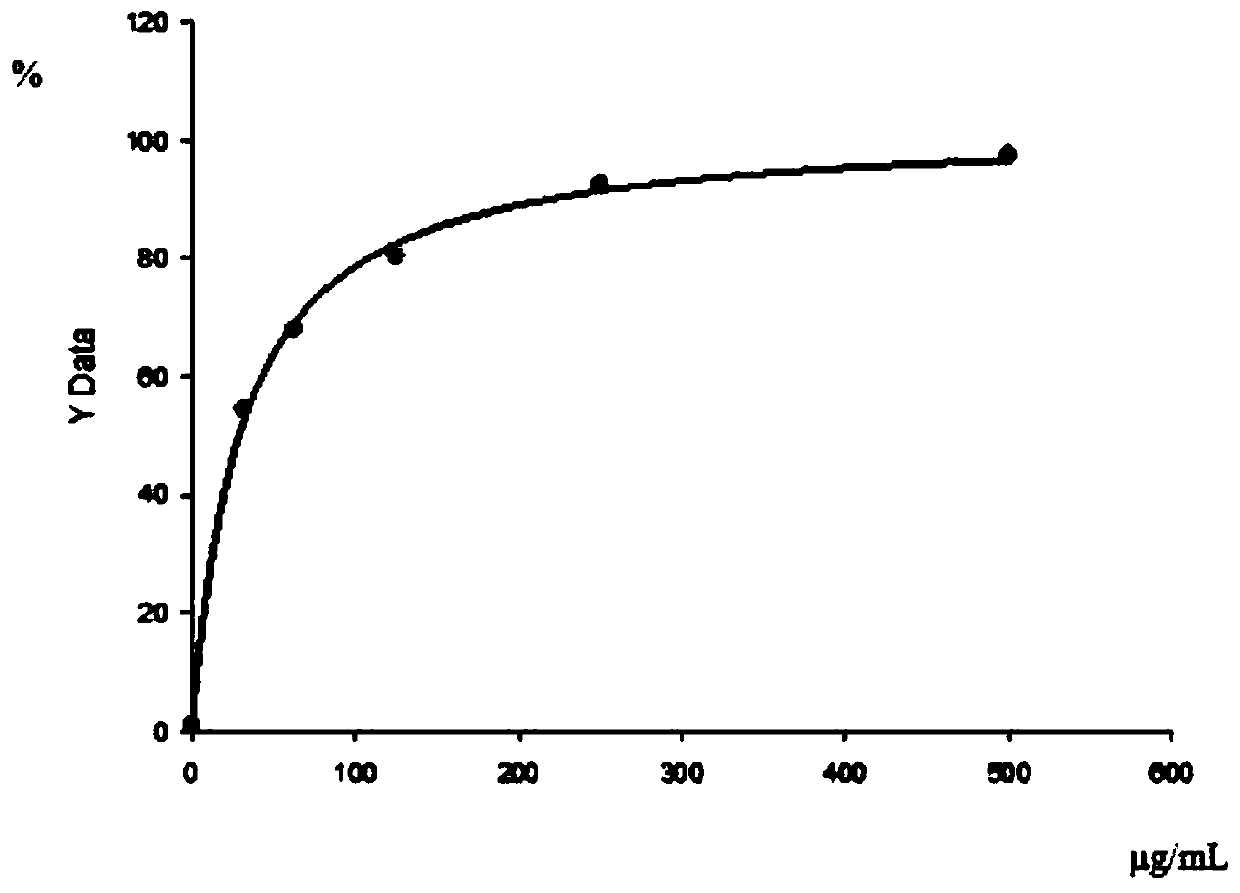
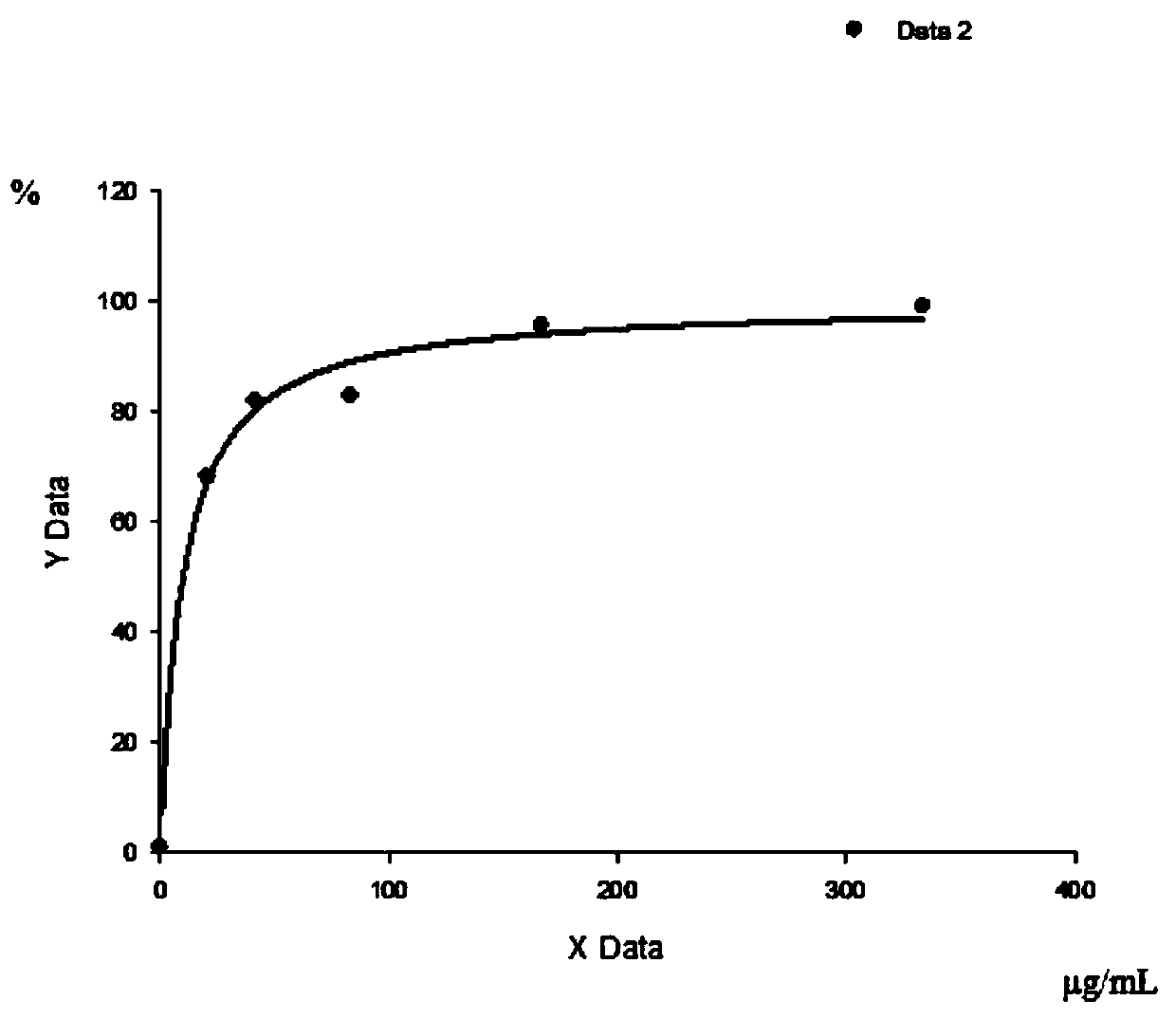
[0072] 3. Binding curve of KRAS mutant polypeptide to HLA on PBMC
[0073] 1) Take 30mL PBMC and centrifuge at 2000rpm for 5min;
[0074] 2) Cells were resuspended in 20mL PBS and counted, centrifuged at 2000rpm for 5min, resuspended in 1.5mL PBS to make the cell concentration 2×10 7 cells / mL;
[0075] 3) Take 50 μL cells (10 6 1) Add 0, 3.2, 6.3, 12...
Embodiment 2
[0081] Example 2 Loading DC with Mixed Antigen
[0082] 1) Blood collection and separation of PBMC;
[0083] 2) Adjust the PBMC to 1×10 medium 1640+10% FBS 6 Cells / mL, as in Petri dish, at 37°C 5% CO 2 Incubator, rest overnight;
[0084] 3) Pipette the cells attached to the bottom of the culture dish with culture medium X-VIVO+1% human serum albumin to collect the adherent monocytes; count and adjust to 1×10 6 Cells / mL, as for the dish, add 1000IU / mL IL-4 and 800IU / mL GM-CSF, at 37℃5%CO 2 incubator;
[0085] 4) After 3 days of culture, mature DCs were collected by centrifugation, re-selected with 5 mL KRAS mutant polypeptide mixture, and kept at 37°C in 5% CO 2 Incubator, impact 12h;
[0086] 5) Centrifuge at 2000 rpm for 5 minutes, collect DC, and resuspend in OKM100+12% FBS for use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com