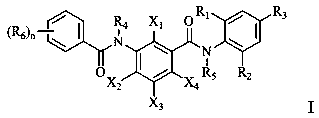
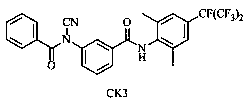
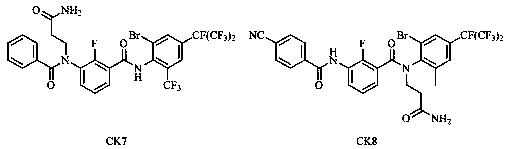
Benzamide compound and application thereof
A technology of benzamide and compound, applied in the field of benzamide compounds, can solve problems such as unreported insecticidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0161] Embodiment 1: the preparation of compound 2.321
[0162]
[0163] Add 0.30 g (7.5 mmol) of 60% sodium hydride to 10 ml of tetrahydrofuran, drop in 0.50 g (0.75 mmol) of 3-benzamido-N-(2,6-dibromo- 4-Heptafluoroisopropylphenyl)-2-fluorobenzamide (intermediate II-1), stirred at room temperature for 10 minutes; added 0.45 g (3.78 mmol) of bromoacetonitrile, and continued to stir at room temperature for 1 hour. After the completion of the reaction monitored by TLC, the reaction was quenched with ice water, extracted with ethyl acetate, dried over anhydrous magnesium sulfate, filtered, and precipitated under reduced pressure. ratio of 1:6~1:2) to obtain 0.38 g of white solid.
[0164] The NMR and mass spectrum data of compound 2.321 are as follows:
[0165] 1 H NMR (600 MHz, internal standard TMS, solvent CDCl 3 ) δ(ppm): 8.10 (t, 1H), 7.98 (d, 1H),7.86 (s, 2H), 7.54 – 7.48(m, 1H), 7.41 – 7.29(m, 4H), 7.28 – 7.21(m , 2H),4.80 (d, 2H).
[0166] 1 H NMR (600MHz, inte...
Embodiment 2
[0181] Embodiment 2: the preparation of compound 3.321
[0182]
[0183] From 3-benzamido-N-(2,6-dichloro-4-heptafluoroisopropylphenyl)-2-fluorobenzamide (intermediate II-2, prepared according to the method reported in WO2010018857) The method for preparing compound 3.321 is the same as that in Example 1.
[0184] The NMR and mass spectrum data of compound 3.321 are as follows:
[0185] 1 H NMR (600MHz, internal standard TMS, solvent CDCl 3 ) δ(ppm): 8.10 (t, 1H), 7.95 (d, 1H),7.67 (s, 2H), 7.49 (t, 1H), 7.41 – 7.34 (m, 3H), 7.31 (t, 1H), 7.29 – 7.22(m, 2H), 4.80 (d, 2H).
[0186] 1 H NMR (600MHz, internal standard TMS, solvent DMSO) δ(ppm): 10.61 (s, 1H), 7.92 (s, 2H),7.70 – 7.58 (m, 2H), 7.42 – 7.27 (m, 6H), 4.93 (s, 2H).
[0187] LC-MS (m / z): 610.0 (m+H).
Embodiment 3
[0188] Embodiment 3: the preparation of compound 6.321
[0189]
[0190] Prepared from 3-benzamido-N-(2-bromo-6-methyl-4-heptafluoroisopropylphenyl)-2-fluorobenzamide (intermediate II-3, referring to the method reported in WO2010018857 Obtained) The method for preparing compound 6.321 is the same as that in Example 1.
[0191] The NMR and mass spectrum data of compound 6.321 are as follows:
[0192] 1 H NMR (600MHz, internal standard TMS, solvent CDCl 3 ) δ(ppm): 8.12-8.06 (m, 1H), 7.90 (d,1H), 7.72 (s, 1H), 7.51 (t, 1H), 7.51 (t, 1H), 7.48 (s, 1H), 7.42 – 7.30 (m, 4H), 7.28 – 7.22 (m, 1H), 4.81 (d, 2H), 2.38 (s, 3H).
[0193] 1 H NMR (600MHz, internal standard TMS, solvent DMSO) δ(ppm): 10.29 (s, 1H), 7.80 (d, 1H),7.70 – 7.64 (m, 2H), 7.59 (t, 1H), 7.38 (d , 3H), 7.35 – 7.27 (m, 3H), 4.93(s, 2H), 2.35 (s, 3H).
[0194] LC-MS (m / z): 634.0 (m+H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com