Alkaline hydroxyl phenazine organic flow battery and preparation method thereof
A technology of hydroxyphenazine and liquid flow battery, which is applied in the field of electrochemical energy storage, can solve the problem of high decay rate of the negative electrode, and achieve the effect of good voltage adjustability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] An alkaline hydroxyphenazine organic liquid flow battery, the preparation method of which is as follows:
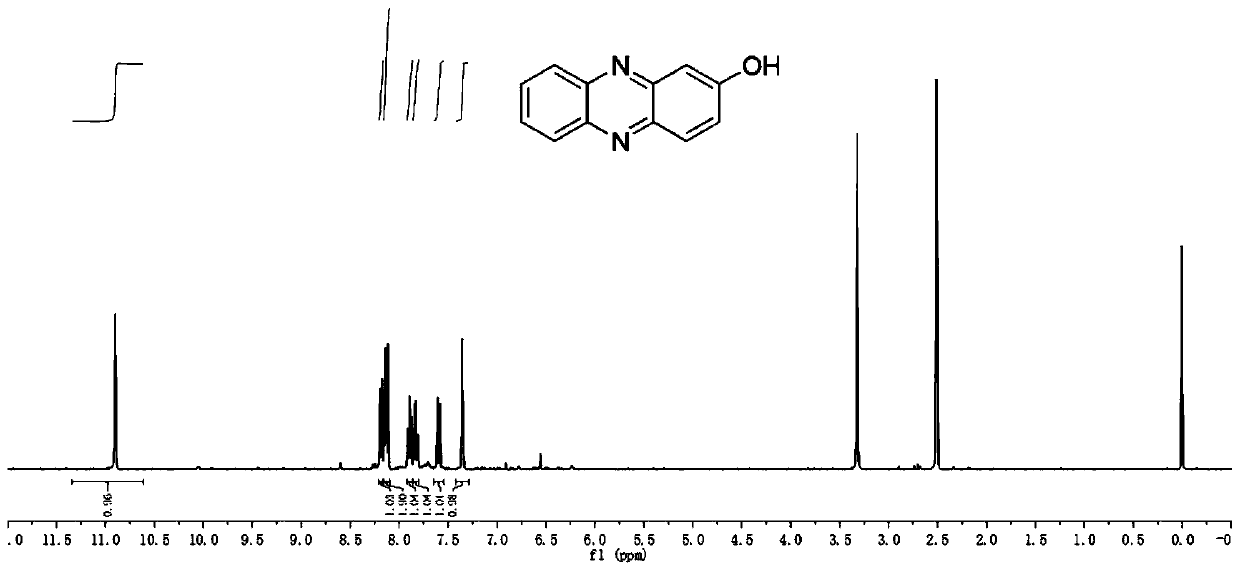
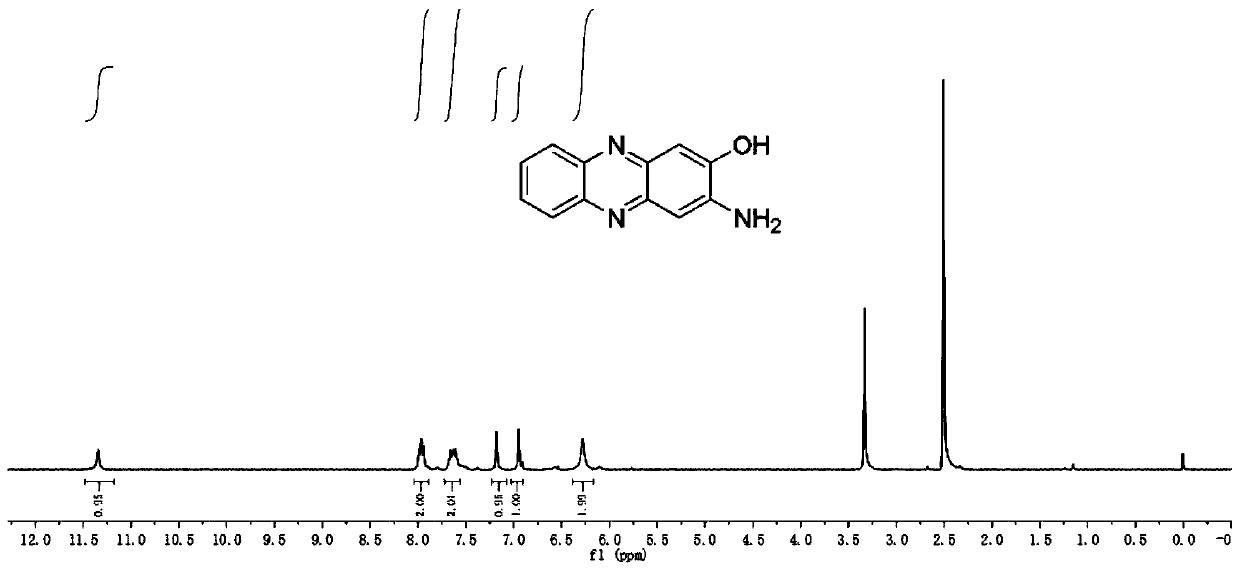
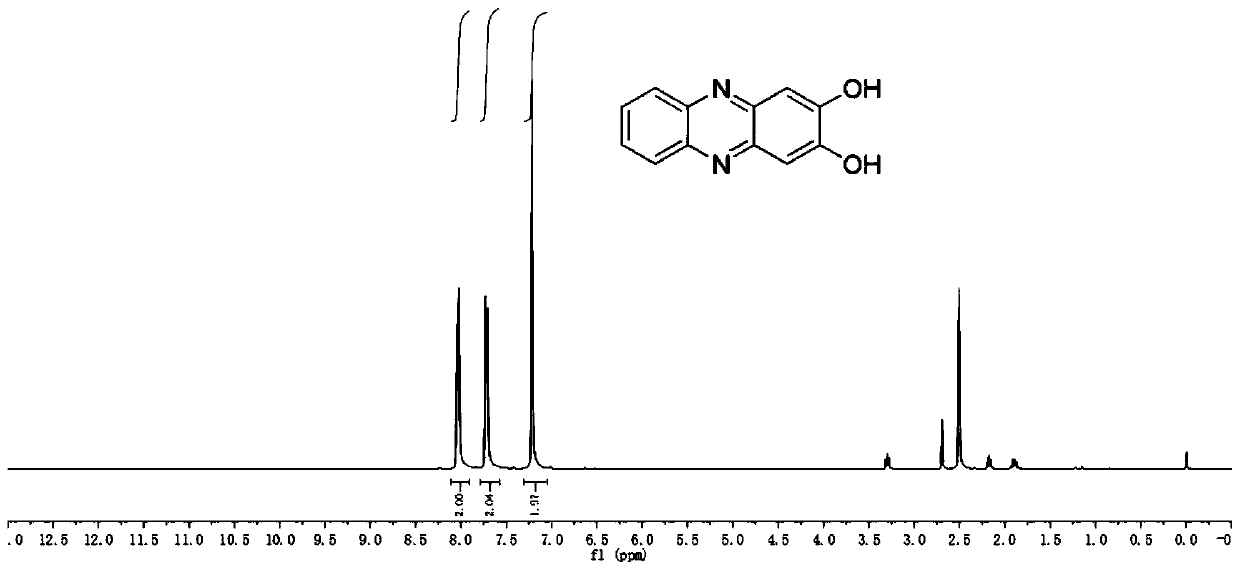
[0046] like Figure 1-4 As shown, the preparation of hydroxyphenazine is as follows:
[0047] Benzoquinone and its derivatives and o-phenylenediamine are reacted in a low temperature environment with absolute ethanol as a solvent for 2-12 hours. After the reaction, a large amount of water is added to filter, the filter cake is collected, and dried to obtain HP and its derivatives.
[0048]Use ammonium persulfate as a catalyst and water as a solvent to react o-phenylenediamine and its derivatives for 12-48 hours. After the reaction, add an alkali solution to filter, collect the filtrate, and adjust the pH of the filtrate so that the AHP and its derivatives in the filtrate are After the solid is precipitated, it is suction filtered, the filter cake is collected, and then dried to obtain AHP and its derivatives.
[0049] Reflux reaction of 2,5-dihydroxybenzoquinone ...
Embodiment 2
[0061] An alkaline hydroxyphenazine organic liquid flow battery, the preparation method of which is as follows:
[0062] Hydroxyphenazine was prepared as follows:
[0063] Benzoquinone and its derivatives and o-phenylenediamine were reacted in a low temperature environment with absolute ethanol as a solvent for 8 hours. After the reaction, a large amount of water was added to filter, the filter cake was collected, and dried to obtain HP and its derivatives.
[0064] Use ammonium persulfate as a catalyst and water as a solvent to react o-phenylenediamine and its derivatives for 24 hours. After the reaction, add an alkali solution to filter, collect the filtrate, and adjust the pH of the filtrate so that AHP and its derivatives in the filtrate are precipitated as solids. After suction filtration, the filter cake is collected, and then dried to obtain AHP and its derivatives.
[0065] Reflux reaction of 2,5-dihydroxybenzoquinone and its derivatives with o-phenylenediamine and it...
Embodiment 3
[0072] An alkaline hydroxyphenazine organic liquid flow battery, the preparation method of which is as follows:
[0073] Hydroxyphenazine was prepared as follows:
[0074] Benzoquinone and its derivatives and o-phenylenediamine were reacted in a low temperature environment with absolute ethanol as a solvent for 10 hours. After the reaction, a large amount of water was added to filter, the filter cake was collected, and dried to obtain HP and its derivatives.
[0075] Use ammonium persulfate as a catalyst and water as a solvent to react o-phenylenediamine and its derivatives for 20 hours. After the reaction, add an alkali solution to filter, collect the filtrate, and adjust the pH of the filtrate so that AHP and its derivatives in the filtrate are precipitated as solids. After suction filtration, the filter cake is collected, and then dried to obtain AHP and its derivatives.
[0076] Reflux reaction of 2,5-dihydroxybenzoquinone and its derivatives with o-phenylenediamine and i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com