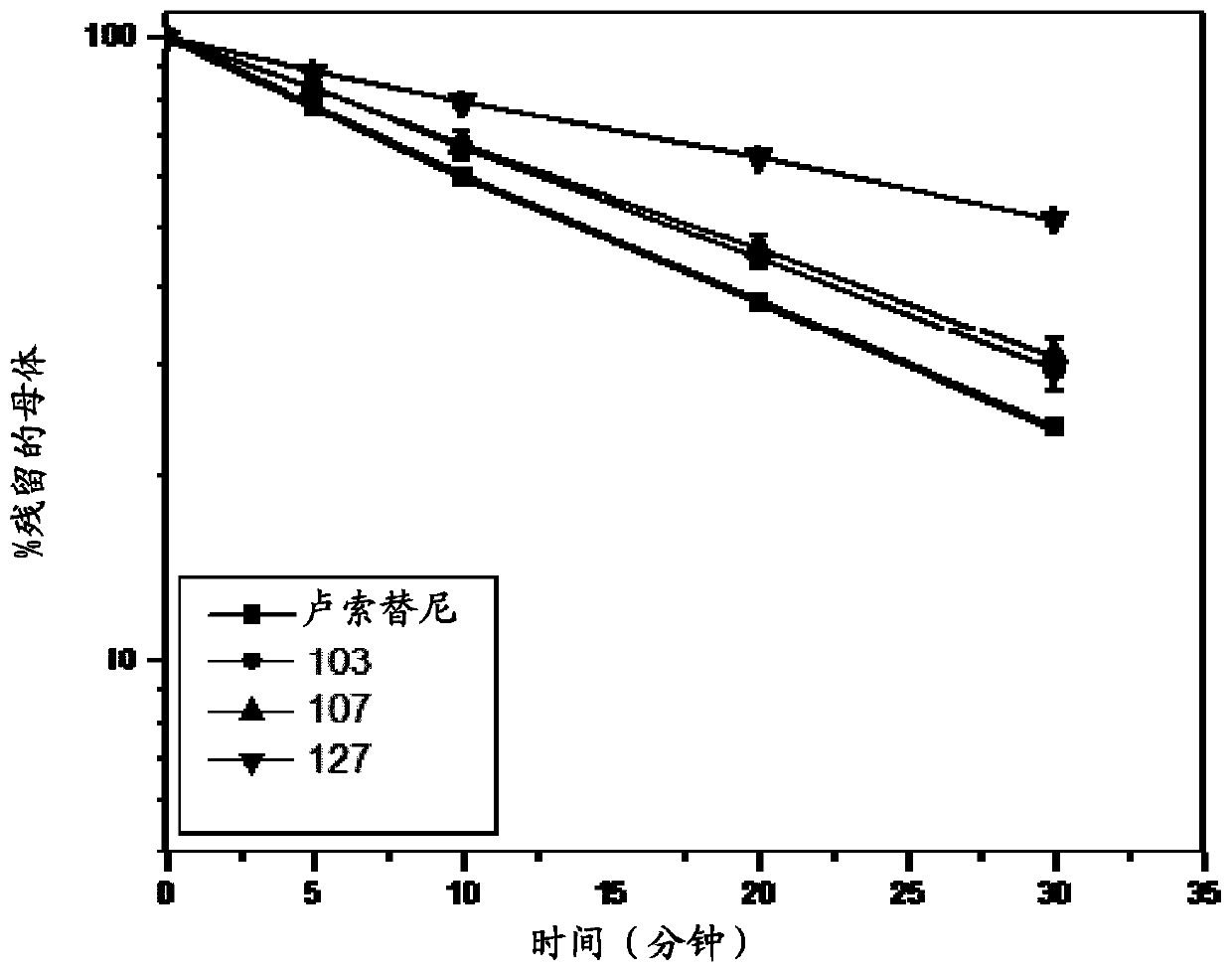
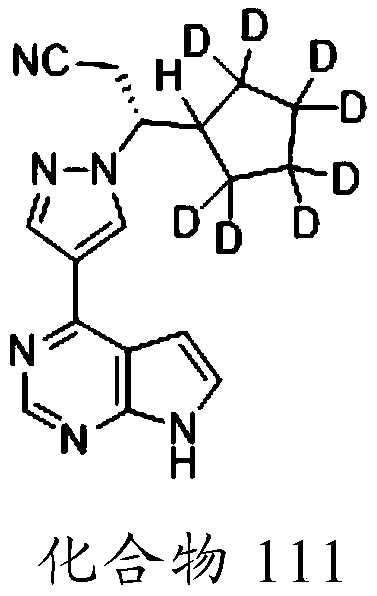
Deuterated derivative of ruxolitinib
A drug and representative technology, applied in the field of deuterated derivatives of ruxolitinib, which can solve the problems of reduced metabolic clearance, variability, and unpredictability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0091]
[0092]
[0093]
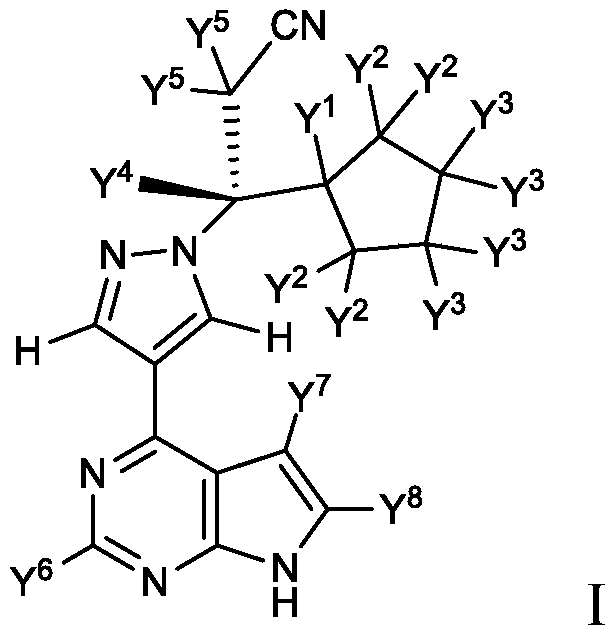
[0094] or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present in its natural isotopic abundance.
[0095] In one embodiment, the compound is a compound of formula I, wherein Y 6 , Y 7 and Y 8 Each is deuterium, and the compound is selected from any compound (Cmpd) listed in Table 2 (below):
[0096] Table 2: Exemplary Embodiments of Formula I
[0097]
[0098]
[0099] or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present in its natural isotopic abundance.
[0100] In another set of embodiments, any atom not designated as deuterium in any of the above embodiments is present in its natural isotopic abundance.
[0101] The following compounds are useful in the preparation of various compounds of the invention:
[0102]
[0103] as well as
[0104] or a salt thereof, wherein any atom not designated as deuterium is present in its n...
Embodiment 1
[0170] Example 1. (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-(2,2,5 ,5-d 4 -Synthesis of cyclopentyl)propionitrile (compound 107).
[0171] Scheme 3. Preparation of compound 107
[0172]
[0173]
[0174] Step 1. 2,2,5,5-d 4 -Diethyl cyclopentane-1,1-dicarboxylate (32) . To a solution of diethyl malonate (6.57mL, 43.3mmol) in ethanol (40mL) was added 21% by weight sodium ethoxide in ethanol (32.3mL, 86.6mmol), followed by the addition of 1,1,4,4-tetra Deuterium-1,4-dibromobutane (31, 5.53 mL, 45.5 mmol, CDN Isotopes, 98 atomic % deuterium). The resulting solution was stirred at reflux for 2 hours, then cooled to room temperature and diluted with excess water. Most of the ethanol was then removed by distillation, and the resulting aqueous solution was extracted with ethyl acetate (3 x 75 mL). The organic layers were combined, washed with brine, and dried (Na 2 SO 4 ), filtered and concentrated under reduced pressure to give 32 as a yellow oil,...
Embodiment 2
[0183] Example 2. (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-(3,3,4 ,4-d 4 -Synthesis of cyclopentyl)propionitrile (Compound 103).
[0184] Scheme 4. Preparation of compound 103
[0185]
[0186] Step 1. 3,3,4,4-d 4 -Diethyl cyclopentane-1,1-dicarboxylate (40) . To a solution of diethyl malonate (3.25 mL, 21.4 mmol) in ethanol (20 mL) was added 21% by weight sodium ethoxide in ethanol (16.0 mL, 42.8 mmol) followed by 2,2,3,3-Tetra Deutero-1,4-dibromobutane (39, 4.95 g, 22.5 mmol, CDN Isotopes, 98 atomic % deuterium). The resulting solution was stirred at reflux for 2 hours, then cooled to room temperature and diluted with excess water. Most of the ethanol was then removed by distillation, and the resulting aqueous solution was extracted with ethyl acetate (3 x 75 mL). The organic layers were combined, washed with brine, and dried (Na 2 SO 4 ), filtered and concentrated under reduced pressure to give 40 as a yellow oil, which was carried on to th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com