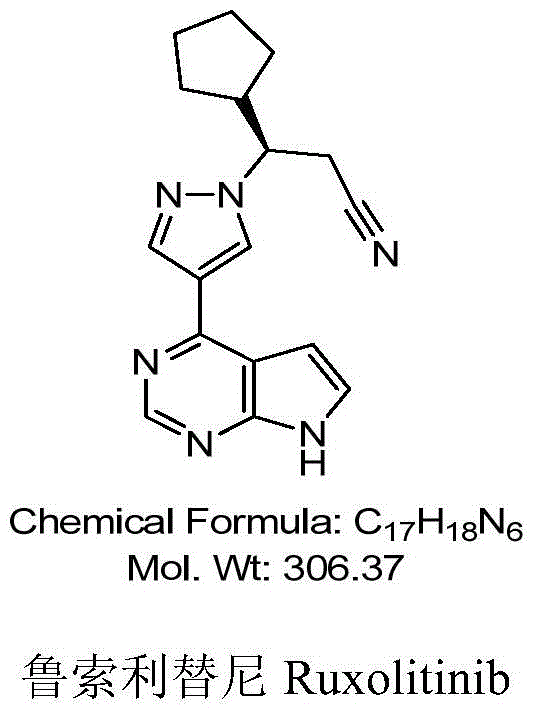
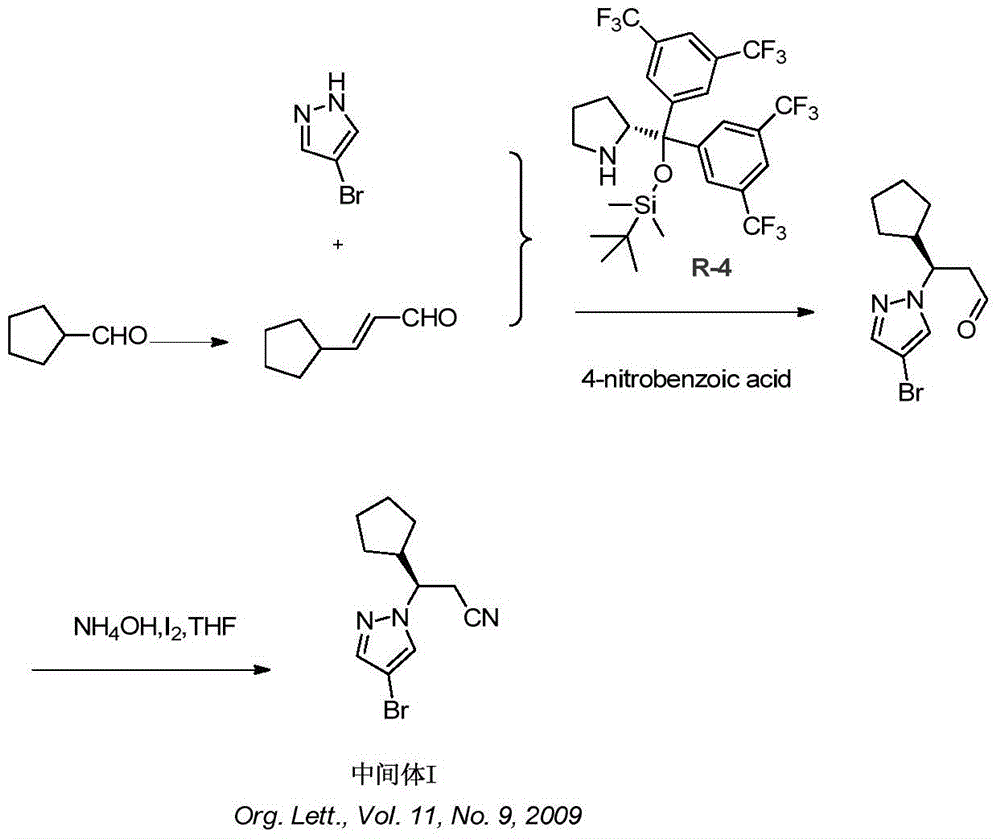
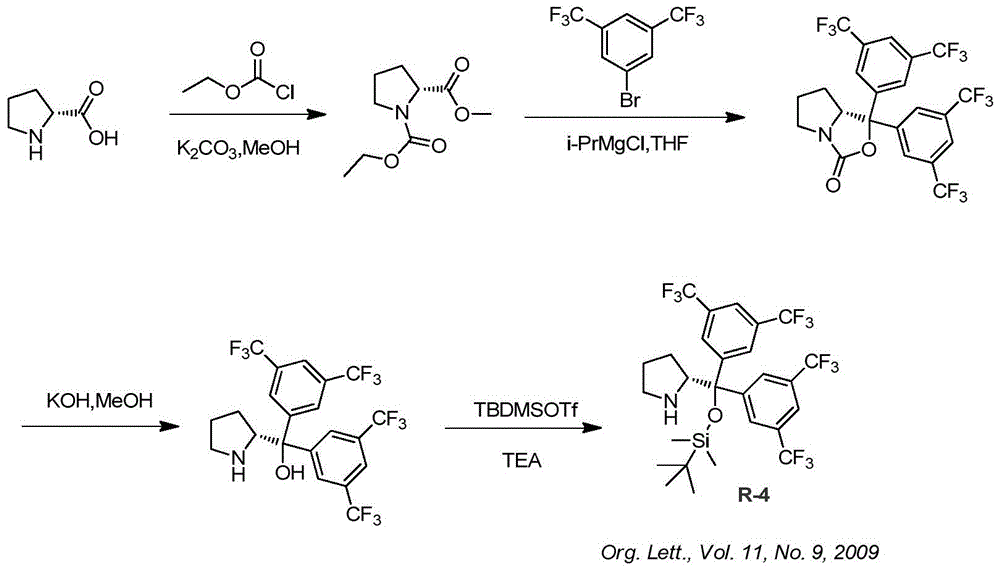
A kind of synthetic method of ruxolitinib intermediate
A ruxolitinib and synthetic method technology, applied in the direction of organic chemistry, etc., can solve the problems of unsuitability for industrialized large-scale production, poor stereoselectivity, long synthesis route, etc., and achieve stable and controllable reaction, mild conditions, and reaction. The effect of fewer steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] 1) Preparation of 3-cyclopentyl-3-oxopropionitrile (Ⅱ)
[0060] 8.95 g (78.07 mmol) of 35 wt% potassium hydride was added to 40 mL of tetrahydrofuran, and heated to 60°C. 10 g (78.07 mmol) of methyl cyclopentacarboxylate was dissolved in 3.2 g (78.07 mmol) of acetonitrile, and slowly added dropwise to the reaction solution of potassium hydride. After completion of the dropwise addition, react at 60°C for 6 hours, cool, concentrate to remove part of the solvent, add 60 mL of water, extract three times with ethyl acetate, and discard the organic phase. The aqueous phase was acidified with 1M hydrochloric acid to pH = 1, extracted three times with ethyl acetate, dried, filtered, and the filtrate was rotary evaporated to obtain 9.63 g of bright yellow oil 3-cyclopentyl-3-oxopropionitrile (II). The yield was 90%.
[0061] 1 H-NMR (400MHz, DMSO-d 6 ): 4.10(2H,s), 3.00(1H,m), 1.90(8H,m).
[0062] 2) Preparation of (S)-3-cyclopentyl-3-hydroxypropionitrile (Ⅲ)
[0063] Add...
Embodiment 2
[0069] 1) Preparation of 3-cyclopentyl-3-oxopropionitrile (Ⅱ)
[0070] 14.4 g (0.36 mol) of 60% sodium hydride was added to 175 mL of tetrahydrofuran, and heated to 80°C. 23.1 g (0.18 mol) of methyl cyclopentacarboxylate was dissolved in 14.8 g (0.36 mol) of acetonitrile, and slowly added dropwise to the reaction solution of sodium hydride. After the dropwise addition, react at 80°C for 20 hours, cool, concentrate to remove part of the solvent, add 160 mL of water, extract three times with ethyl acetate, and discard the organic phase. The aqueous phase was acidified with 6M sulfuric acid to pH=5, extracted three times with ethyl acetate, dried, and rotary evaporated to obtain 22.45 g of bright yellow 3-cyclopentyl-3-oxopropionitrile (II), with a yield of 91%.
[0071] NMR data is the same as in Example 1.
[0072] 2) Preparation of (S)-3-cyclopentyl-3-hydroxypropionitrile (Ⅲ)
[0073] Add D-glucose (7g), D-glucose dehydrogenase (0.28g), NADPH (reduced coenzyme II) (0.42g) a...
Embodiment 3
[0079] 1) Preparation of 3-cyclopentyl-3-oxopropionitrile (Ⅱ)
[0080] Add 24.4 g (0.61 mol) of 60% sodium hydride into 335 mL of tetrahydrofuran, and heat to 75°C. 50 g (0.39 mol) of methyl cyclopentacarboxylate was dissolved in 25 g (0.61 mol) of acetonitrile, and slowly added dropwise to the reaction solution of sodium hydride. After the dropwise addition, react at 70°C for 15 hours, cool, concentrate to remove part of the solvent, add 180 mL of water, extract three times with ethyl acetate, and discard the organic phase. The aqueous phase was acidified with 4M hydrochloric acid to pH = 2, extracted three times with ethyl acetate, dried, and rotary evaporated to obtain 49.75 g of bright yellow 3-cyclopentyl-3-oxopropionitrile (II), with a yield of 93%.
[0081] Nuclear magnetic data is with embodiment 1.
[0082] 2) Preparation of (S)-3-cyclopentyl-3-hydroxypropionitrile (Ⅲ)
[0083] Add D-glucose (22.7g), D-glucose dehydrogenase (750mg), NADPH (reduced coenzyme II) (750...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com