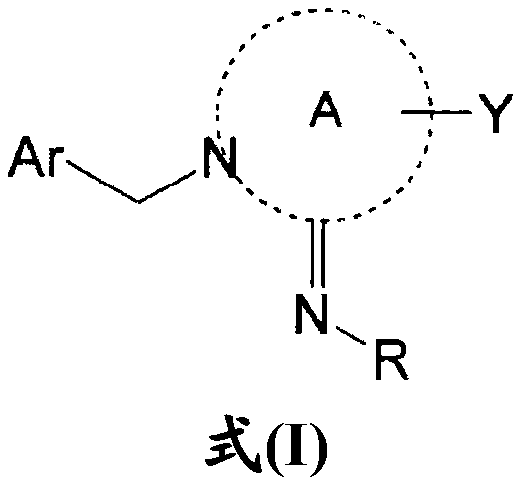
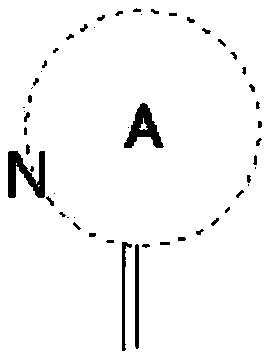
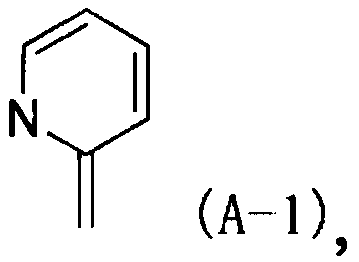
Nitrogen-containing heterocyclic derivative having 2-imino group and pest control agent including the same
A pest control, imino technology, applied in biocides, insecticides, animal repellents, etc., can solve the problems of drug sensitivity and safety reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0868] Next, the present invention is described more specifically with reference to Examples, but the present invention is not limited to the Examples.
[0869] Reference Example 1: N-[1-((6-chloropyridin-3-yl)methyl)pyridin-2(1H)-ylidene]-2,2,2-trifluoroacetamide (compound P212)
[0870] (1) 25 g (270 mmol) of 2-aminopyridine was dissolved in 200 mL of anhydrous dichloromethane, 41 mL (30 g, 300 mmol) of triethylamine was added, and the mixture was cooled to 0°C. 38 mL (57 g, 270 mmol) of anhydrous trifluoroacetic acid was added dropwise over 15 minutes, and the resulting mixture was stirred at room temperature for 2 hours. After the reaction was completed, the reaction solution was poured into about 100 mL of ice water, and the mixture was stirred for 10 minutes. The mixture was moved to a separatory funnel for liquid separation, and the organic layer was washed twice with 150 mL of water and twice with 150 mL of 1% aqueous HCl, dried over anhydrous magnesium sulfate, and c...
preparation Embodiment 1
[1191] Preparation Example 1 [Granules]
[1192]
[1193] The above ingredients are uniformly ground and mixed, water is added to knead the ingredients well, and the mixture is then granulated and dried to obtain granules.
preparation Embodiment 2
[1194] Preparation Example 2 [Granules]
[1195]
[1196] The above ingredients are uniformly ground and mixed, water is added to knead the ingredients well, and the mixture is then granulated and dried to obtain granules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com