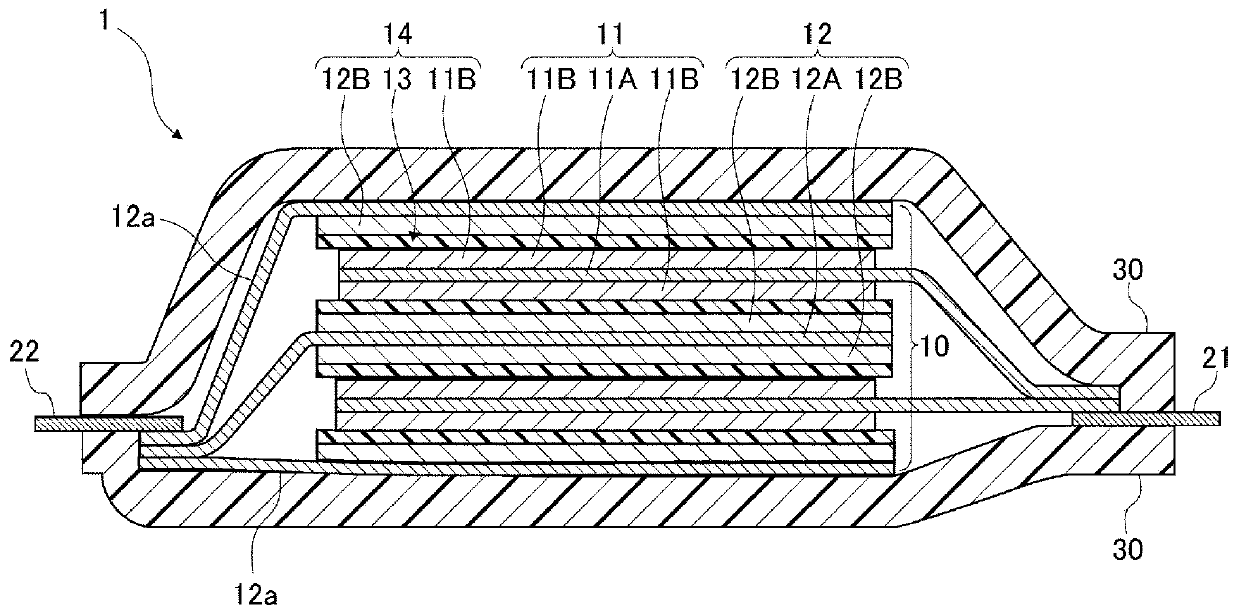
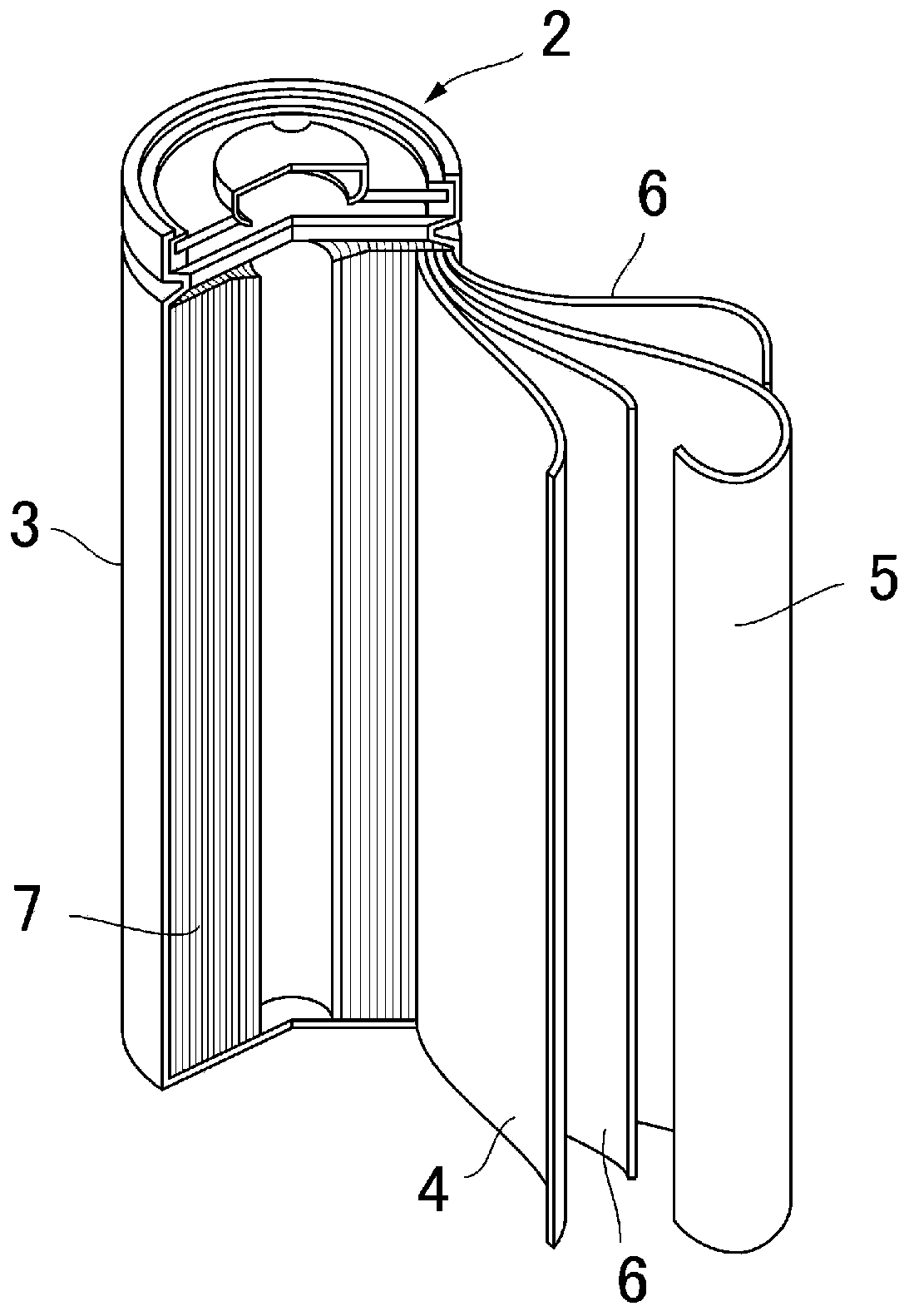
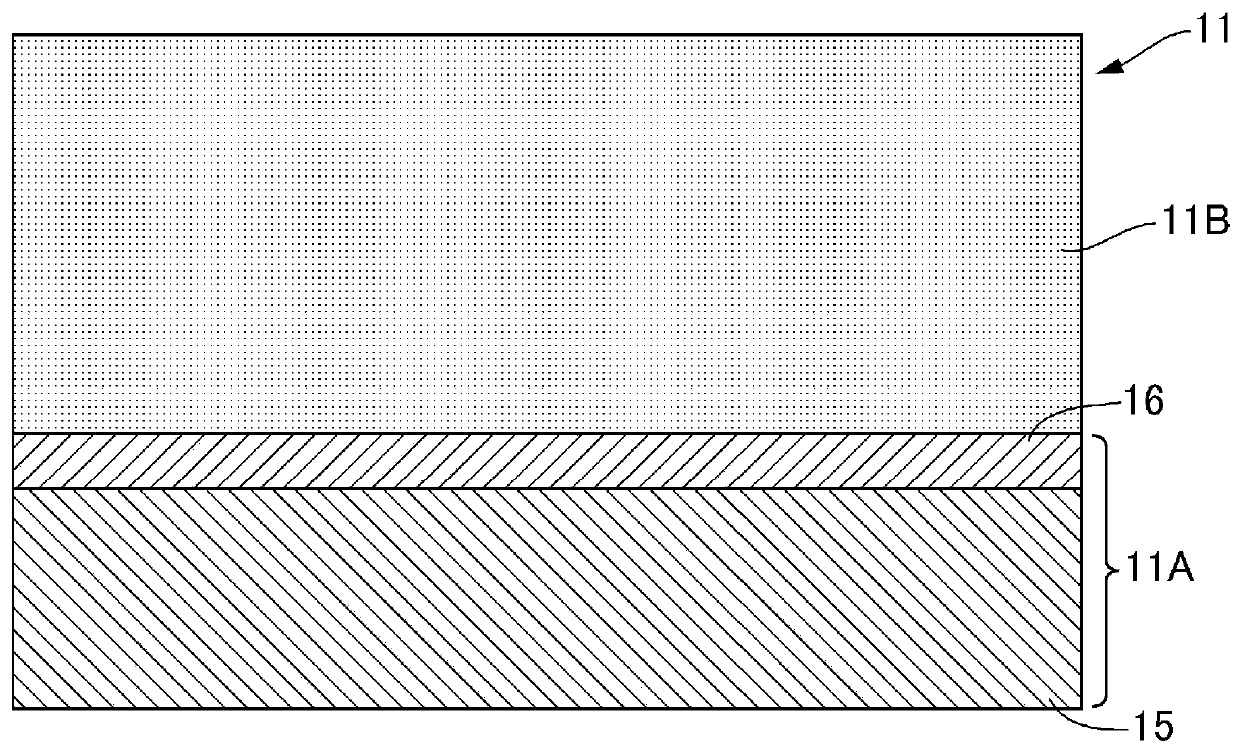
Current collector, electrode, and non-aqueous electrolyte secondary battery
A non-aqueous electrolyte and secondary battery technology, applied in the direction of non-aqueous electrolyte batteries, non-aqueous electrolyte battery electrodes, secondary batteries, etc., to achieve the effect of increasing resistance and suppressing thermal runaway
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0163] Production of PTC layer
[0164] 1. Slurry Preparation
[0165] For slurry preparation, a 5 L planetary mixer was used.
[0166] 900 g of flaky graphite (manufactured by TIMICAL, with an average particle diameter of 2 μm) and 25 g of Super-P (conductive carbon manufactured by TIMCAL) were mixed for 10 minutes, and then 200 g of N-methylpyrrolidone (NMP) was added, and further carried out for 20 minutes. minutes to mix.
[0167] Next, 62.5 g of a mixed liquid (BX1) obtained by dissolving 40 g of a mixture of BMI-1000 / BTA=2 / 1 in 1,000 g of NMP, and 8%-PVDF solution (dissolving PVDFW#7200 manufactured by KUREHA in NMP )62.5g, NMP1000g, kneading for 60 minutes. The solid content concentration at this time was 43% (1000 g of solid content, 1317.5 g of NMP).
[0168] Furthermore, NMP 1000g was added, and kneading was performed for 60 minutes (30% of solid content concentration).
[0169] Then, in order to adjust the viscosity, 682.5 g of NMP was added and mixed for 30 ...
Embodiment 2~24
[0207] It should be noted that in Table 6, the PTC layers shown in PTC2 to 24 were produced in the following manner: according to Example 1, the mixture with the PTC function-imparting component and PVDF (PVDFW#7200 manufactured by KUREHA) were replaced with Except the combination shown in Table 6, it carried out similarly to Example 1, and in Examples 2-24, positive electrodes C-2-24 which arrange|positioned these PTC layers were produced.
Embodiment 25~28
[0209] In addition, in Table 6, the PTC layers shown in PTC-25 to PTC-28 were prepared as follows: According to Example 1, instead of the mixture with the PTC function-imparting component and PVDF (KUREHA PVDFW#7200), The PTC layer was fabricated using a binder liquid obtained by dissolving PVDF-HFP copolymer in NMP.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com