Synthesis method of teneligliptin key intermediate
A synthesis method and a technology for ticagliptin are applied in the field of synthesis of key intermediates of ticagliptin, which can solve the problems of low overall yield, difficult removal, influence on yield and the like, achieve cheap and easy-to-obtain raw materials, and reduce complexity. , the effect of simple and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
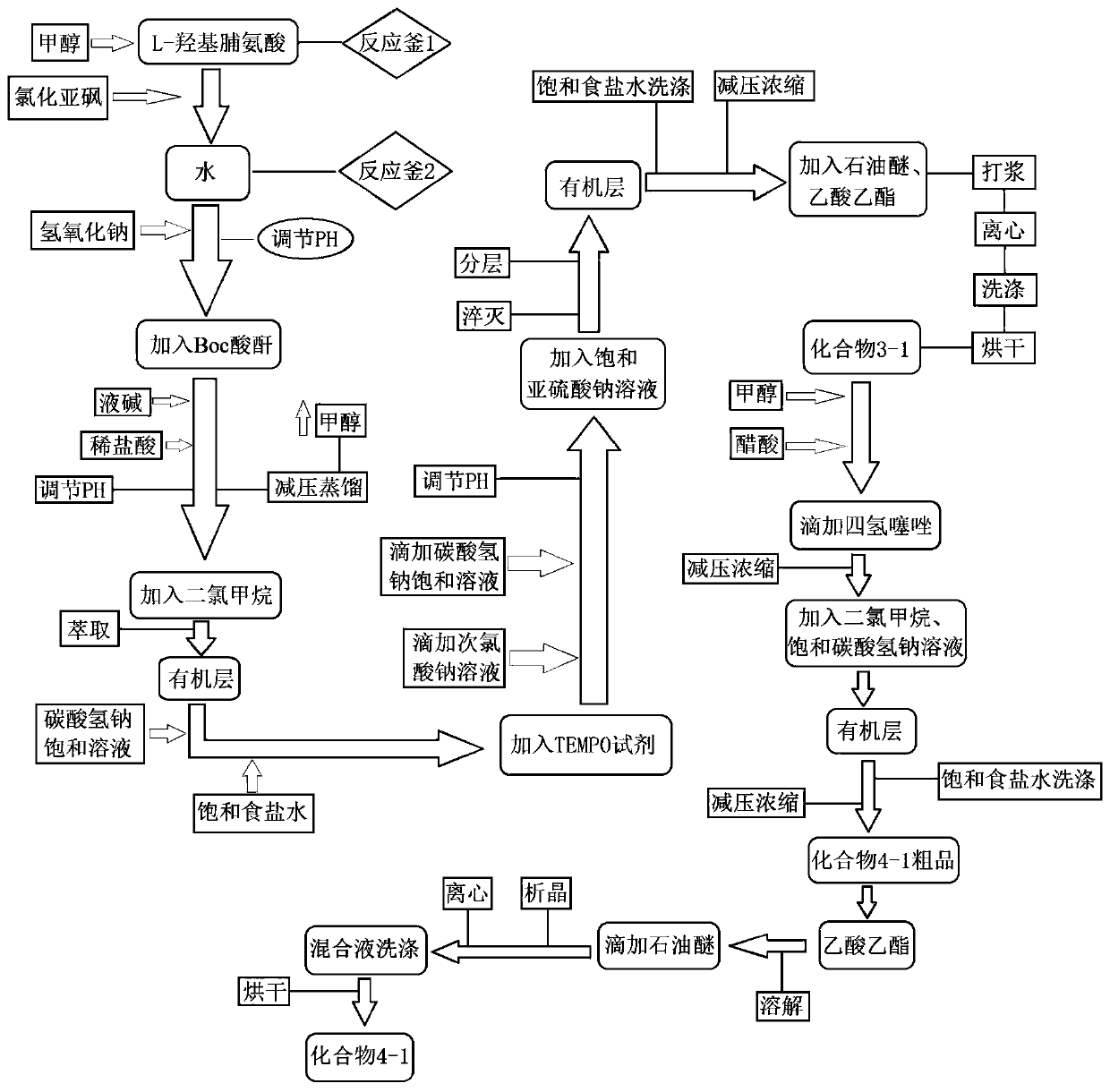
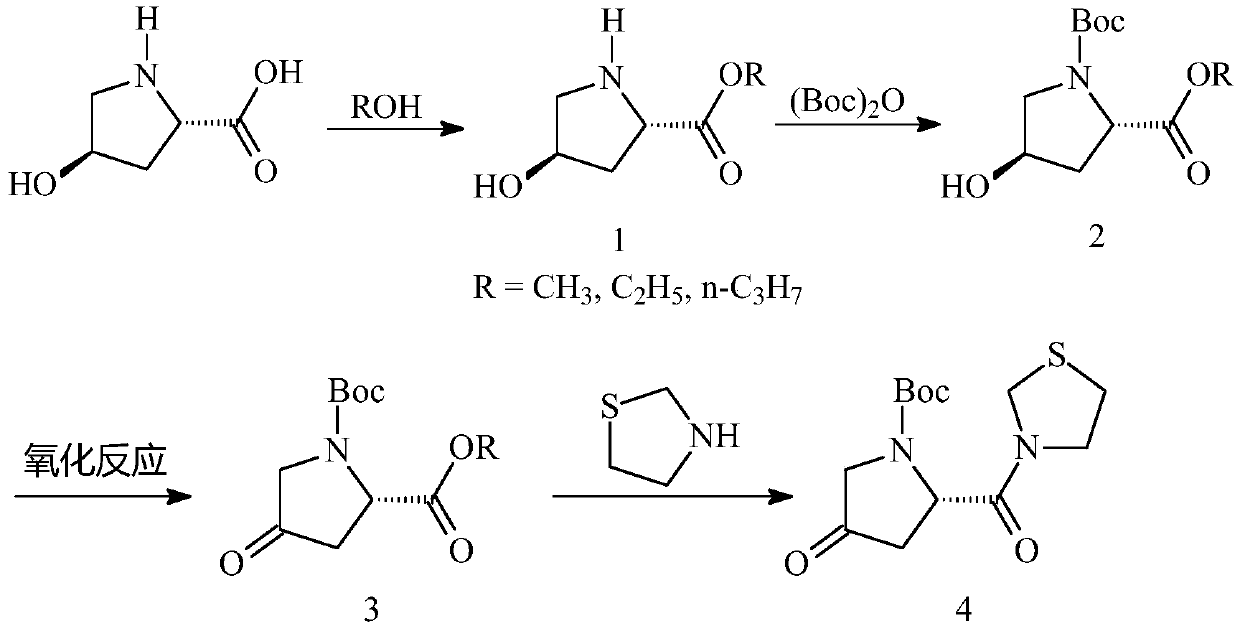
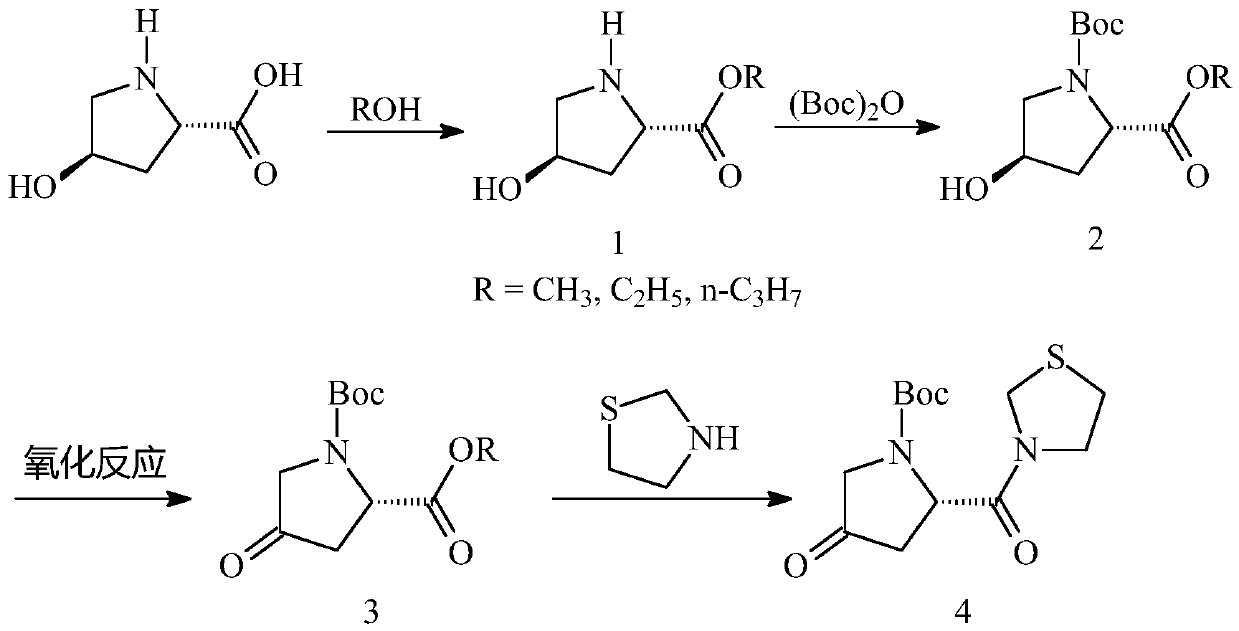
[0021] In order to make the object, technical solution and advantages of the present invention clearer, the present invention will be further described in detail below in conjunction with the accompanying drawings and embodiments. It should be understood that the specific embodiments described here are only used to explain the present invention, not to limit the present invention.
[0022] Specific synthetic process one, compound 1-1 is synthesized:
[0023]
[0024] The specific method is as follows:
[0025] Add 500Kg of methanol and 150Kg of L-hydroxyproline into the reaction kettle, lower the temperature to -10~0°C, then add 162Kg of thionyl chloride dropwise, and the internal temperature does not exceed 5°C. After dripping and keeping warm for 2 hours, the central control is qualified. Add 100L of water into another reaction kettle, add the reaction solution dropwise into water to quench, and the internal temperature does not exceed 30°C. Continue to stir for 20-30 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com