Pteridine compounds and application of same in pharmacy
A compound, pteridine technology, applied in the field of medicine, can solve the problems of narrow treatment window and affect the clinical treatment level, and achieve the effect of strong selectivity and broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
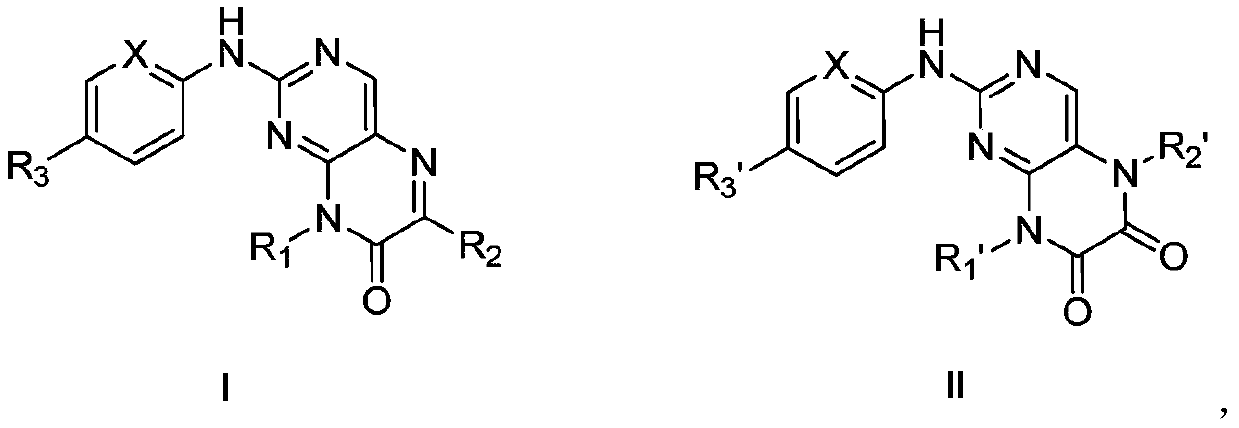
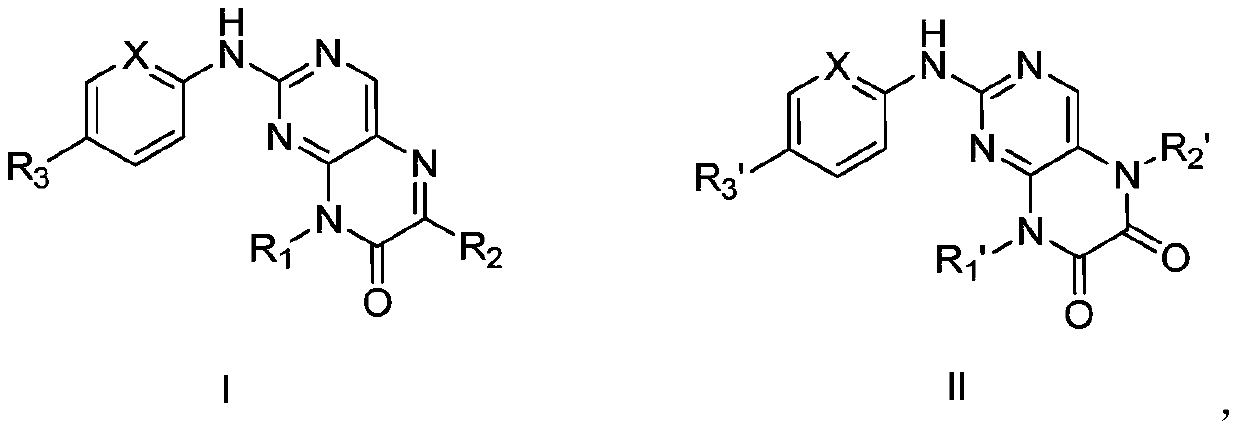
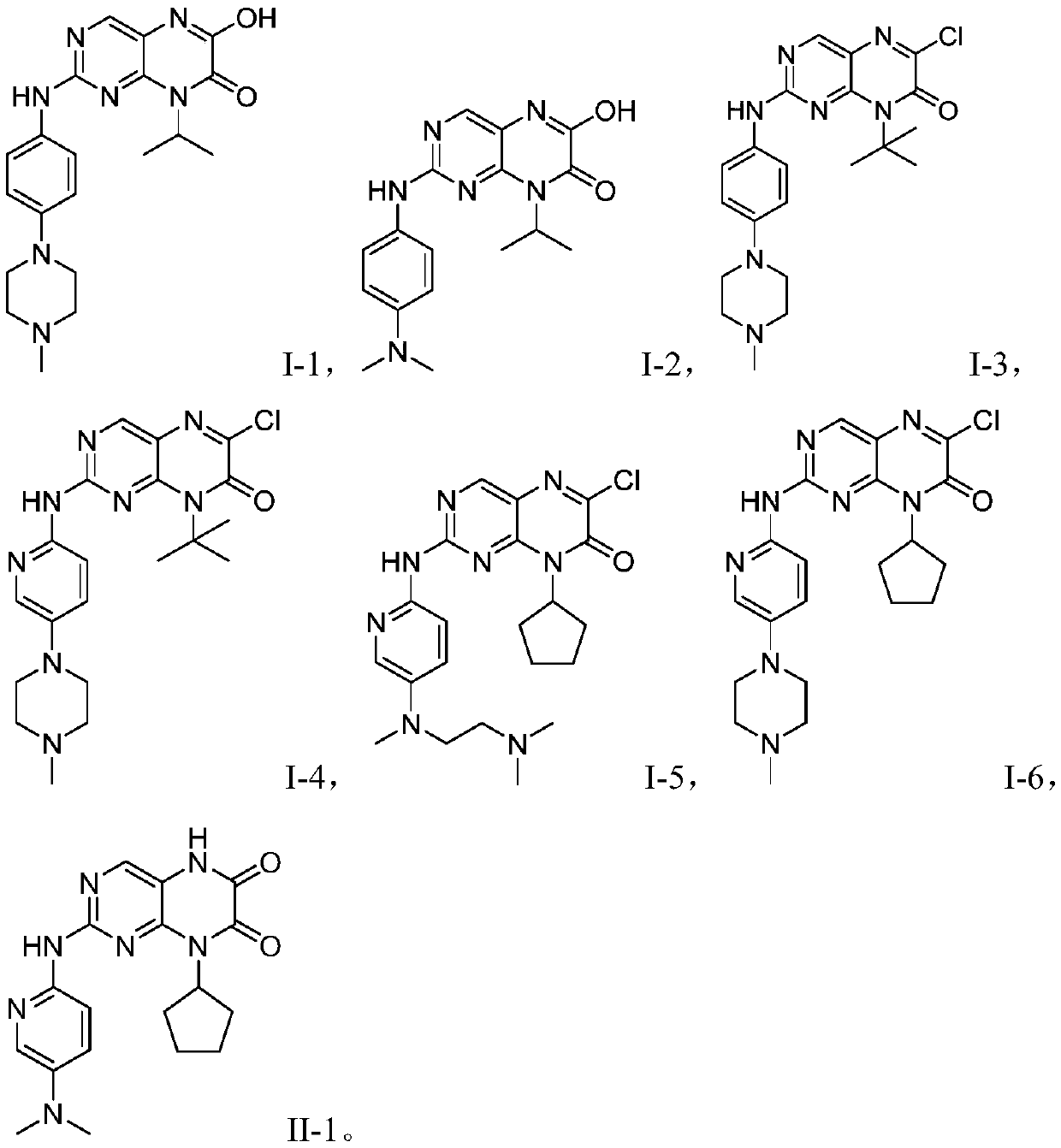
[0038] Example 1 Preparation of Compound I-1
[0039] (1) Intermediate compound 2-chloro-N 4 – Preparation of isopropylpyrimidine-4,5-diamine
[0040]
[0041] 5-Amino-2,4-dichloropyrimidine (25.0 g, 0.15 mol) was dissolved in anhydrous tetrahydrofuran (100 mL), and under stirring at room temperature, triethylamine was added dropwise to the reaction system using a constant pressure dropping funnel The mixed solution of (30.4g, 0.3mol) and isopropylamine (17.7g, 0.3mol) was dripped, and the reaction system was moved to an oil bath at 80° C. to react for 8h. TLC detected that the reaction was complete, and after cooling to room temperature, water (100 mL) was added, extracted with ethyl acetate (100 mL×3), and the organic phases were combined. The organic phase was washed with saturated sodium chloride water, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated and purified by silica gel column chromatography to obtain a reddish-brown soli...
Embodiment 2
[0051] Example 2 Preparation of Compound I-2
[0052]
[0053] With reference to Example 1, 4-(4-methylpiperazine)aniline in step (3) was replaced with equimolar amount of N,N-dimethyl-1,4-phenylenediamine, other conditions were unchanged, Compound I-2 was obtained (yield 72%).
[0054] MS-ESI(m / z): 340.2[M+H]+; 1 H NMR (400MHz, DMSO) δ: 12.57(s,1H), 8.86(s,1H), 7.32(s,1H), 7.00(d,2H), 6.76(d,2H), 3.80(q,1H) ,3.02(s,6H),1.26(d,6H)
Embodiment 3
[0055]Example 3 Preparation of Compound I-3
[0056] (1) Intermediate 2-chloro-N 4 – Preparation of tert-butylpyrimidine-4,5-diamine
[0057]
[0058] 5-Amino-2,4-dichloropyrimidine (0.15mol) was dissolved in anhydrous tetrahydrofuran (100mL), and under stirring at room temperature, triethylamine (30.4g) was added dropwise to the reaction system using a constant pressure dropping funnel. , 0.3mol) and tert-butylamine (21.9g, 0.3mol) mixed solution, after dripping, the reaction system was moved to 100 ℃ oil bath to react for 16h. TLC detected that the reaction was complete, and after cooling to room temperature, water (100 mL) was added, extracted with ethyl acetate (100 mL×3), and the organic phases were combined. The organic phase was washed with saturated sodium chloride water, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated and purified by silica gel column chromatography to obtain a reddish-brown solid (21.0 g, yield 69.8%). MS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com