Synthesis method of paranitroaniline
A technology for p-nitroaniline and a synthesis method, which is applied in the field of synthesis of p-nitroaniline, can solve problems such as low production efficiency, unstable product quality, poor equipment amplification reliability, etc., so as to improve production efficiency, improve technical economy, The effect of compressing the recycling cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
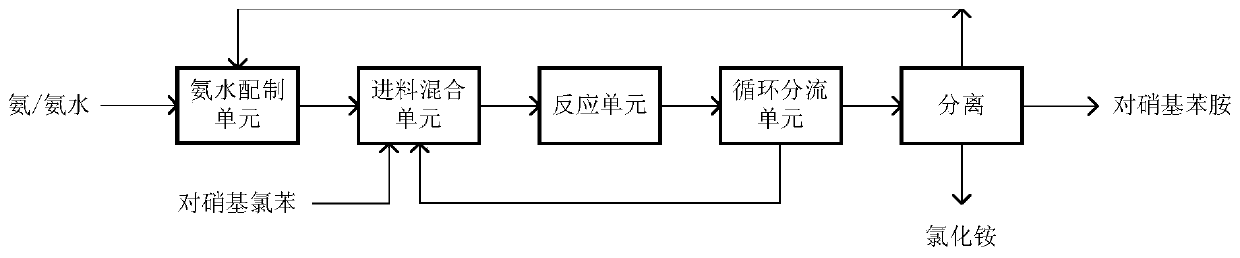
[0028] The ammonia water preparation unit prepares the ammonia water with a concentration of 25wt%, and the p-nitrochlorobenzene that melts at 120° C. is transported into the feed mixing unit respectively, and the molar ratio of ammonia water and p-nitrochlorobenzene is 4; the feed mix unit uses column Tubular heat exchanger and static mixer, exchange the heat of the materials to be mixed to 190°C for mixing, the pressure is 6.2MPa; the temperature of the reaction unit is 190°C, the pressure is 6.0MPa, and the residence time is 4h; the circulation ratio of the split circulation unit is 10. The circulating material enters the feed mixing unit, and is mixed with ammonia water and p-nitrochlorobenzene in sequence. The effluent material is flashed to recover unreacted ammonia, and the p-nitroaniline is obtained through cooling and crystallization, and the by-product ammonium chloride is obtained through evaporation and crystallization. , the mother liquor enters the ammonia water p...
Embodiment 2
[0030] The ammonia water preparation unit prepares the ammonia water with a concentration of 34wt%, and the p-nitrochlorobenzene melted at 120°C is transported into the feed mixing unit respectively, and the molar ratio of the ammonia water and p-nitrochlorobenzene is 20; the feed mixing unit uses Tube-and-tube heat exchanger and static mixer, heat exchange the materials to be mixed to 200°C and mix at a pressure of 7.8MPa; the temperature of the reaction unit is 200°C, the pressure is 7.6MPa, and the residence time is 1.0h; the circulation of the split circulation unit The ratio is 10, the circulating material enters the feed mixing unit, and is sequentially mixed with ammonia water and p-nitrochlorobenzene, and the effluent material is flashed to recover unreacted ammonia, and the p-nitroaniline is obtained through cooling and crystallization, and the by-product chlorine is obtained through evaporation and crystallization. ammonium chloride, and the mother liquor enters the a...
Embodiment 3
[0032] The ammonia water preparation unit prepares the ammonia water with a concentration of 20wt%, and the p-nitrochlorobenzene melted at 120°C is transported into the feed mixing unit respectively, and the molar ratio of the ammonia water and p-nitrochlorobenzene is 18; the feed mixing unit uses Tube-and-tube heat exchanger static mixer, heat exchange the materials to be mixed to 175°C for mixing, the pressure is 3.4MPa; the temperature of the reaction unit is 175°C, the pressure is 3.2MPa, and the residence time is 5h; the circulation ratio of the split circulation unit is 12. The circulating material enters the feed mixing unit, and is mixed with ammonia water and p-nitrochlorobenzene in sequence. The effluent material is flashed to recover unreacted ammonia, and the p-nitroaniline is obtained through cooling and crystallization, and the by-product ammonium chloride is obtained through evaporation and crystallization. , the mother liquor enters the ammonia water preparation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com