Methanesulfonamide derivatives and their preparation methods and their application in the prevention and treatment of Alzheimer's disease
A technology of methanesulfonamide and derivatives, which is applied in the field of Alzheimer's disease, methanesulfonamide derivatives and its preparation, can solve problems such as difficult to improve the cognitive function of patients, and protect nerves from damage, prepare The method is simple and easy to reduce the effect of inflammatory cytokines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
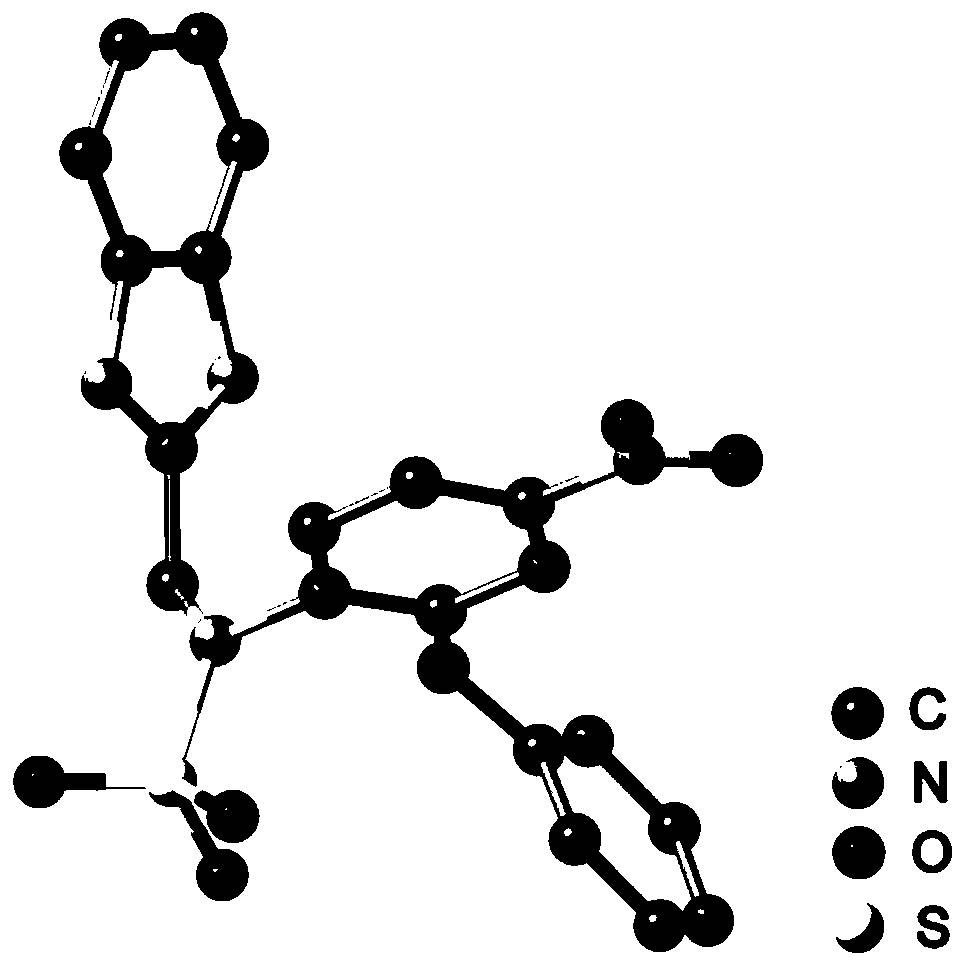
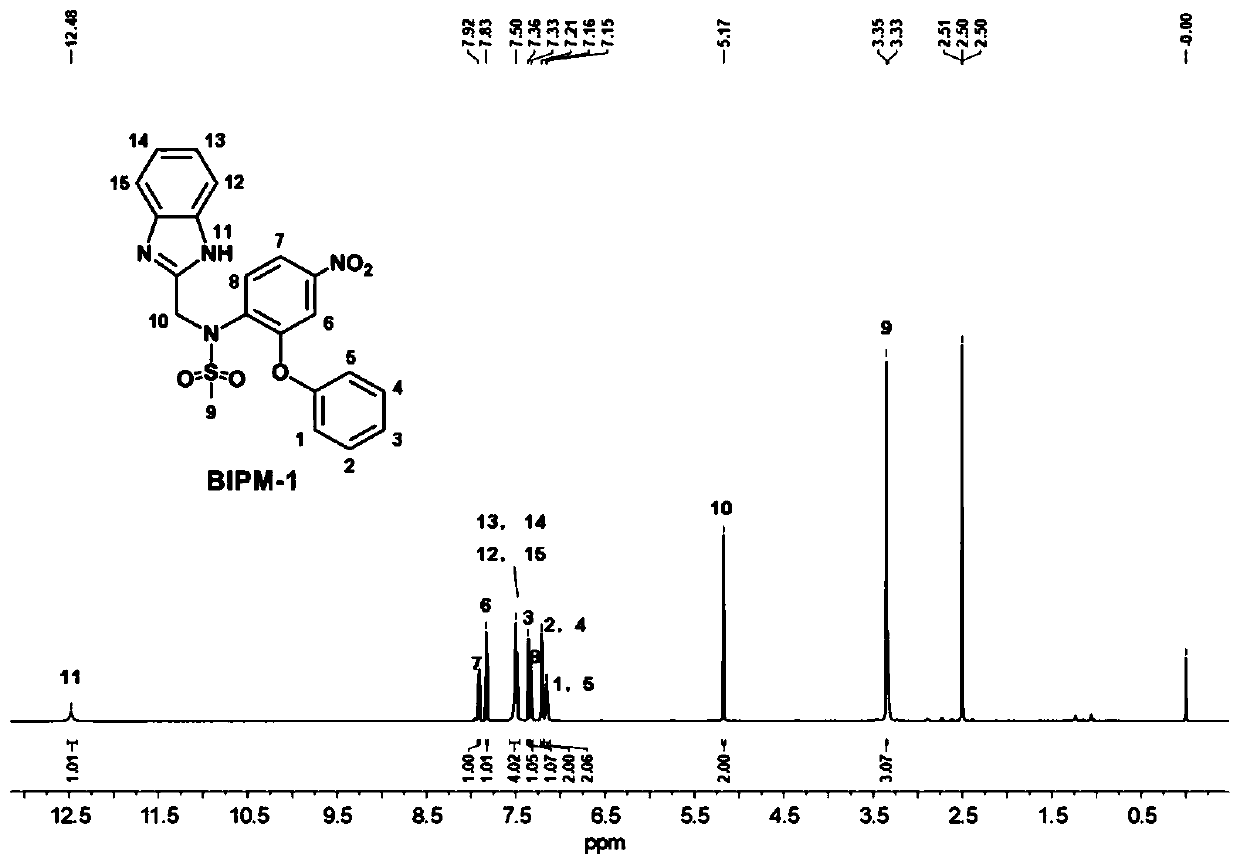
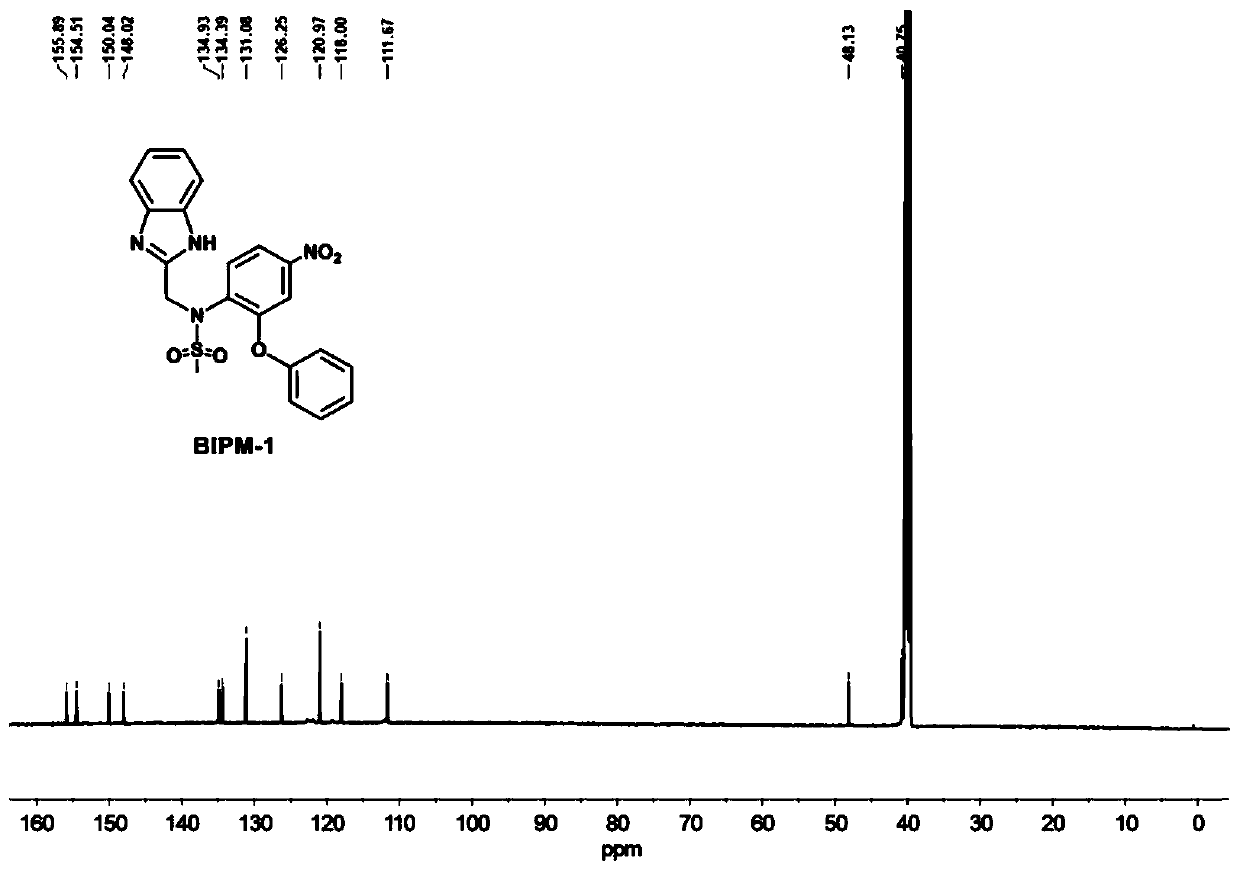
[0031] The preparation of embodiment 1N-((1H-benzo [d] imidazol-2-yl) methyl) -N-(4-nitro-2-phenoxyphenyl) methanesulfonamide (BIPM-1) compound
[0032]
[0033] S1, N-(4-nitro-2-phenoxyphenyl)methanesulfonamide (0.308g, 1mmol) was dissolved in anhydrous DMF solution (8mL), and potassium carbonate was added, under N 2 Under protection, stir at room temperature for 15 min;
[0034] S2. Continue to add a DMF solution of 2-(chloromethyl)benzimidazole (0.199g, 1.2mmol) and stir at 60°C for 2h, and continue stirring at 25°C overnight;
[0035] S3. After the reaction, the precipitate was removed by filtration, concentrated under reduced pressure, added dropwise to the aqueous solution, and the precipitate was collected by centrifugation. The crude product was separated through a silica gel column to obtain 0.247 g of a yellow powder with a yield of 56%. 1 H NMR (DMSO-d 6 ,600MHz,δ,ppm):12.48(s,1H,-NH-),7.92–7.90(q,1H,Ph),7.83–7.82(d,1H,Ph),7.51–7.48(t,4H,benzene imidazole), 7....
Embodiment 2
[0038] Example 2 Test of the influence of BIPM-1 on the performance of cells
[0039] 1. Anti-inflammatory properties of BIPM-1 at the cellular level
[0040] Operation steps: BV-2 cells (3×10 5 cells / well) were inoculated into 12-well plates (DMEM containing 10% fetal bovine serum), and cultivated at 37°C for 18 h; the original culture medium was removed, and serum-free DMEM medium was added; then, BIPM-1 (20μM, 4‰DMSO) pretreated cells for 10h, added LPS (1μg mL -1 ) or Aβ42 (10 μM) pre-incubated for 6 hours, co-cultivated at 37°C for 8 hours; after the above operations were completed, 500 μL of the cell supernatant culture solution were collected, and centrifuged at 12,000g for 15 minutes at 4°C to collect the supernatant; The supernatant was added to each well of the ELISA plate, and the content of inflammatory factors in the sample was measured by enzyme-linked immunoassay (ELISA). Where LPS refers to lipopolysaccharide, and Aβ42 refers to β-amyloid protein.
[0041] ...
Embodiment 3B
[0048] Example 3 Behavioral Test of BIPM-1 on AD Animal Model Caenorhabditis elegans CL4176
[0049] 1. Effect of BIPM-1 on paralysis of Aβ transgenic Caenorhabditis elegans
[0050] Operation steps: Aβ expression in muscle of transgenic Caenorhabditis elegans is closely related to progressive paralysis. When the culture temperature increased from 15°C to 25°C, a large amount of Aβ42 accumulated and deposited in the muscle wall cells, causing a paralytic phenotype. In order to study the ability of BIPM-1 to inhibit or delay the paralysis of Caenorhabditis elegans CL4176 induced by Aβ, CL4176 nematodes were transferred to NGM culture dishes containing Escherichia coli OP50 and Nimesulide or BIPM-1 (20 μM), CL802 nematodes that do not express Aβ42 were used as the control group; let the nematodes lay eggs at 15°C for 2 hours, discard the adult worms, and grow at 15°C for about 36 hours. Count the paralyzed nematodes 20 hours after the start, during which time all nematodes sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com