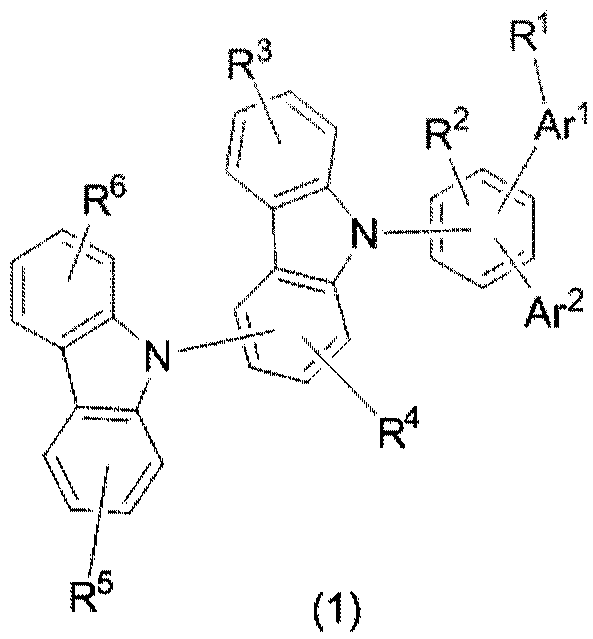
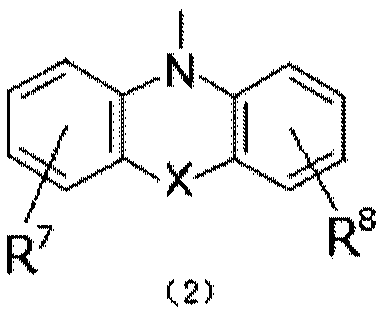
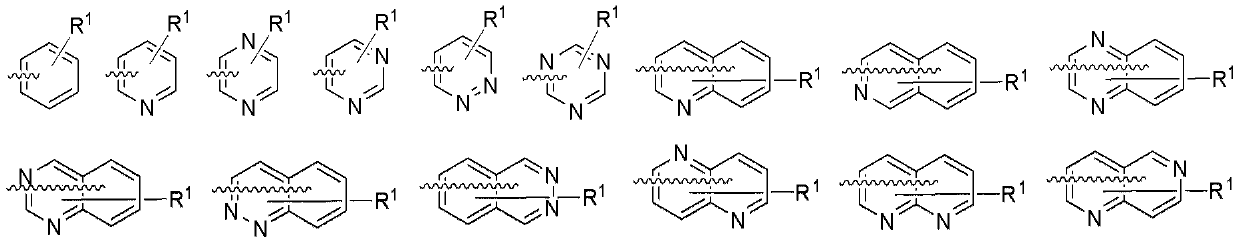
Organic electroluminescent material and device
An organic compound, selected technology, applied in the direction of luminescent materials, electric solid devices, electrical components, etc., can solve the problems of uneven distribution of excitons, unsatisfactory device life, unstable phosphine groups, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0079] Synthesis Example 1: Synthesis of Product P1
[0080]
[0081] Synthesis of Intermediate M1
[0082] Under nitrogen atmosphere, 2.2g (10.0mmol, 1eq) of 4-bromo-2,6-difluorobenzonitrile, 1.8g (12.0mmol, 1.2eq) of 4-cyanophenylboronic acid, Pd (PPh 3 ) 4 Add 0.16g (0.1mmol, 1%eq) and 4.1g (30.0mmol, 3eq) of potassium carbonate to a 100mL single-necked flask, add 20mL toluene, 5mL ethanol and 5mL water, heat up to reflux temperature, and react overnight. Using silica gel column chromatography. Obtained M1, mass spectrum: 240.
[0083] Synthesis of intermediate M2
[0084] Under a nitrogen atmosphere, 3.3g (10.0mmol, 1.0eq) of 3,9-bicarbazole, 0.24g (10.0mmol, 1.0eq) of NaHH, and DMF (30mL) were added to a 100mL single-necked bottle, and stirred for 30min in an ice-water bath Afterwards, 2.4 g (10.0 mmol, 1 eq) of the intermediate M1 was added, the temperature was raised to room temperature, and the reaction was carried out overnight. Using silica gel column chroma...
Synthetic example 2
[0087] Synthesis Example 2: Synthesis of Product P12
[0088]
[0089] Synthesis of Intermediate M3
[0090] The reactant 4-bromo-2,6-difluorobenzonitrile was replaced by 2-bromo-4-iodo-5,6-difluorobenzonitrile, and M3 was obtained through the same synthesis method as the intermediate M1 in Synthesis Example 1. , mass spectrum: 319.
[0091] Synthesis of Intermediate M4
[0092] Under nitrogen atmosphere, the intermediate M3 3.2g (10.0mmol, leq), 3,9-bicarbazole 3.7g (11.0mmol, 1.1eq), Pd 2 (dba) 3 0.09g (0.1mmol, 1%eq), and 2.9g (30.0mmol, 3eq) of potassium tert-butoxide were added to a 100mL single-necked flask, then 20mL of xylene was added, the temperature was raised to reflux temperature, and the reaction was carried out overnight. Using silica gel column chromatography. Obtained M4, mass spectrum: 240.
[0093] Synthesis of Product P12
[0094] The reactant 3,9-bicarbazole was replaced by carbazole to react with intermediate M4, and the synthesis method was the...
Synthetic example 3
[0095] Synthesis Example 3: Synthesis of Product P23
[0096]
[0097] Synthesis of Intermediate M5
[0098] The reactant 4-cyanophenylboronic acid was replaced by 4-trifluoromethylphenylboronic acid, and M5 was obtained through the same synthesis method as the intermediate M1 in Synthesis Example 1, mass spectrum: 283.
[0099] Synthesis of Intermediate M6
[0100] The reactant intermediate M1 was replaced by intermediate M5, and M6 was obtained through the same synthesis method as intermediate M2 in Synthesis Example 1, mass spectrum: 595.
[0101] Synthesis of product P23
[0102] The reactant 3,9-bicarbazole was replaced by carbazole to react with intermediate M6, and through the same synthesis method as intermediate M2 in Synthesis Example 1, P23 was obtained, mass spectrum: 742. 1 H NMR (300MHz, CDCl 3 ): 8.60(3H), 8.30(2H), 8.15(2H), 7.98(1H), 7.94(3H), 7.71(2H), 7.66(3H), 7.53(4H), 7.41(2H), 7.36(3H ), 7.33(1H), 7.31(2H), 7.28(3H).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap