Arenobufagin derivatives, preparation method thereof, composition containing the derivatives and application of the derivatives
A technology of sandbuad toxin and its derivatives, which is applied in the direction of drug combination, steroids, and pharmaceutical formulations, and can solve problems such as unsatisfactory physical and chemical properties, low solubility, and high toxicity of sandbuad toxin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
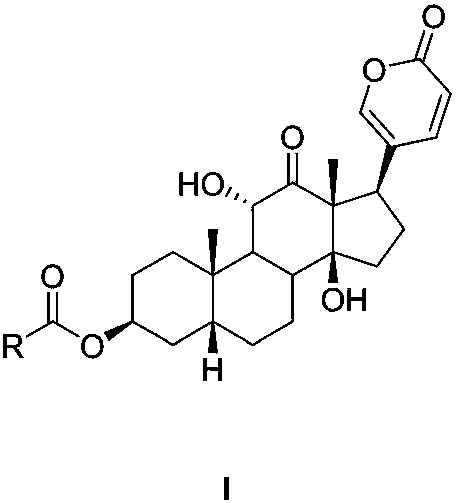
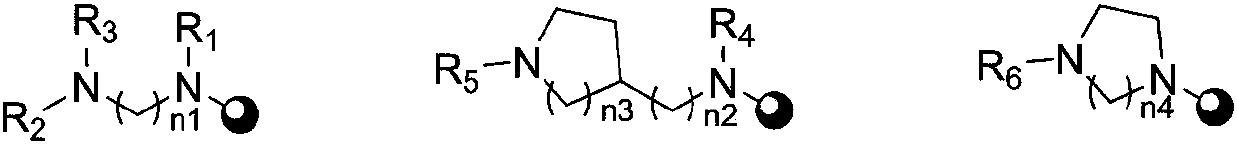
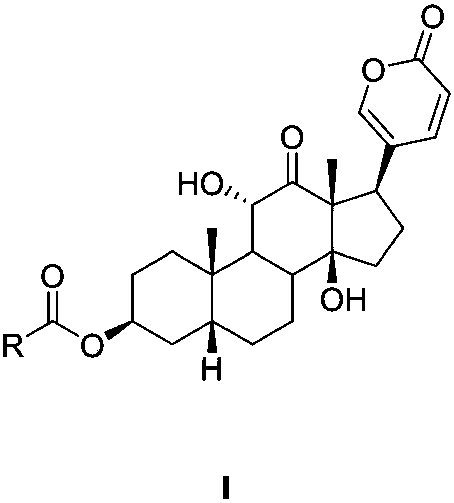
[0055] Preparation Example 1 Sabubuadin-3-N-(2-methylaminoethyl) carbamate (compound 1)
[0056]
[0057] In a 50mL round-bottomed flask, dissolve p-nitrophenyl chloroformate (1.206g, 6mmol) in 10mL of anhydrous dichloromethane, add dry pyridine (0.67mL), a white precipitate appears immediately, and add sand dropwise under nitrogen protection. A dichloromethane solution (10 mL) of bufotoxin (2 mmol) was stirred at room temperature for 6 hours, washed twice with water after the reaction was completed, dried over anhydrous sodium sulfate, concentrated under reduced pressure and subjected to silica gel column chromatography (v:v= 90:10, petroleum ether / acetone) to obtain Intermediate A. In a 10mL round-bottomed flask, dissolve Intermediate A in 3mL of dichloromethane, add triethylamine (35μL), add N-methylethylenediamine (6mmol), stir at room temperature for 2 hours, and use Wash once with saturated sodium carbonate solution, and repeatedly wash with water until the solution ...
preparation Embodiment 2
[0058] Preparation Example 2 Sabubuadin-3-N-(2-ethylaminoethyl) carbamate (compound 2)
[0059]
[0060] The reaction operation is as in the preparation of compound 1, the raw material is N-ethylethylenediamine instead of N-methylethylenediamine; silica gel column chromatography eluent: petroleum ether / acetone / ammonia water (v:v:v=50:50 :0.5), the productive rate is 63%. 1 H NMR (400MHz, CDCl 3 )δ7.71(dd, J=9.8,2.6Hz,1H),7.39(d,J=2.6Hz,1H),6.29(d,J=9.8Hz,1H),4.97(s,1H),4.32( dd,J=11.3,2.4Hz,1H),4.10(dd,J=9.6,7.4Hz,1H),3.82(d,J=3.1Hz,1H),3.38–3.20(m,2H),2.93–2.82 (m,2H),2.81–2.74(m,2H),2.41(d,J=13.3Hz,1H),2.15–1.29(m,15H),1.25(t,J=7.1Hz,3H),1.18( s,3H),0.92(s,3H); ESI-MS(m / z)531.3[M+1] + .
preparation Embodiment 3
[0061] Preparation Example 3 Sabubuadin-3-N-(2-dimethylaminoethyl) carbamate (compound 3)
[0062]
[0063] The reaction operation is the same as the preparation of compound 1, the raw material is N,N-dimethylethylenediamine instead of N-methylethylenediamine; silica gel column chromatography eluent: petroleum ether / acetone / ammonia water (v:v:v= 50:50:0.5), the yield was 61%. 1 H NMR (400MHz, CDCl 3 )δ7.71(dd, J=9.8,2.6Hz,1H),7.39(d,J=2.6Hz,1H),6.29(d,J=9.8Hz,1H),4.97(s,1H),4.32( d,J=11.1Hz,1H), 4.11(d,J=9.3,2.2Hz,1H),3.36–3.21(m,2H),2.50–2.44(m,2H),2.40(d,J=16.3Hz ,1H),2.29(s,6H),2.25–1.21(m,15H)1.18(s,3H),0.92(s,3H); ESI-MS(m / z)531.3[M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com