Diagnostic and therapeutic methods for kras positive cancers
A technology for samples and tumors, applied in the direction of nanotechnology for sensing, instruments, analytical materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
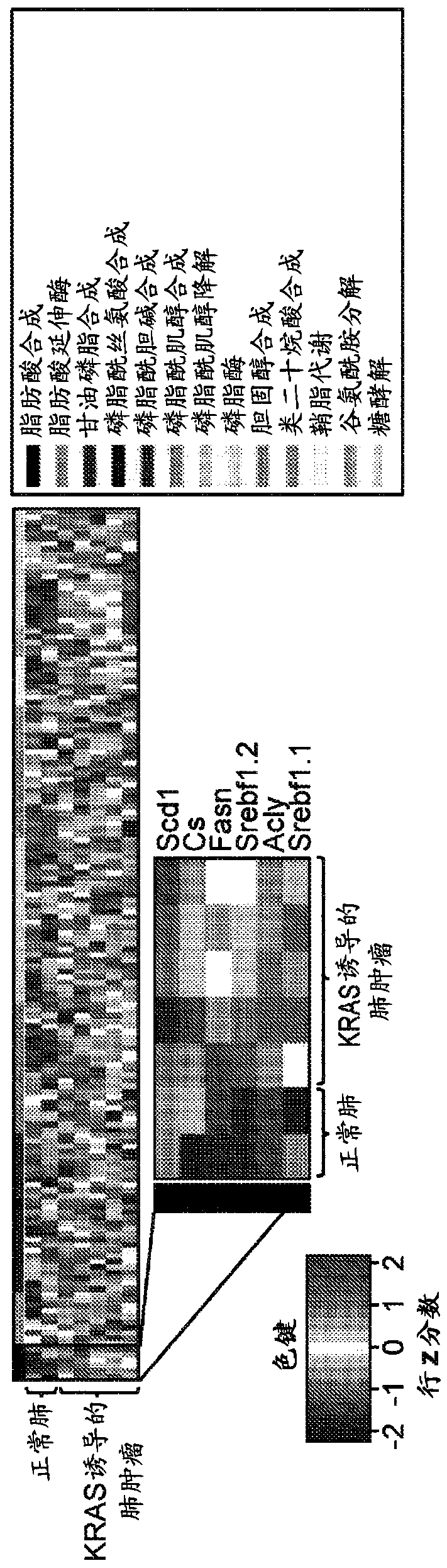
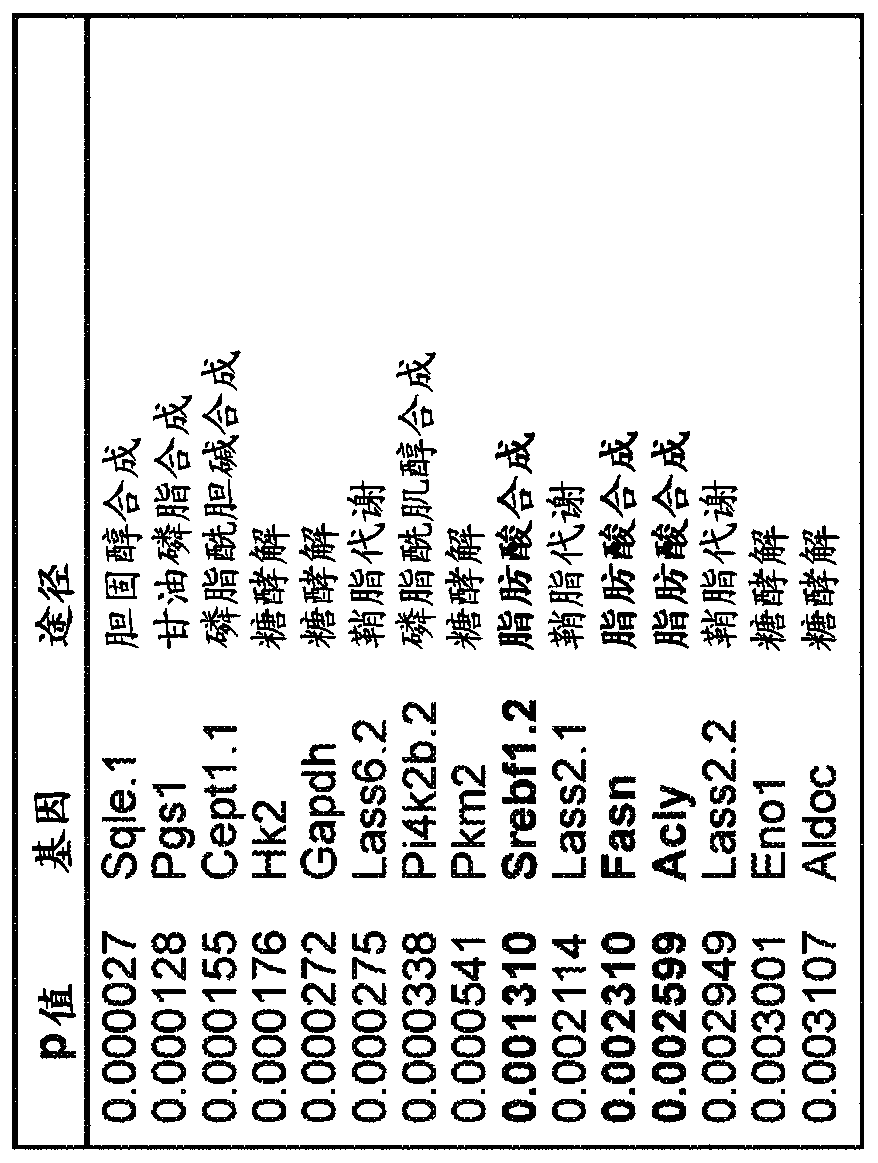
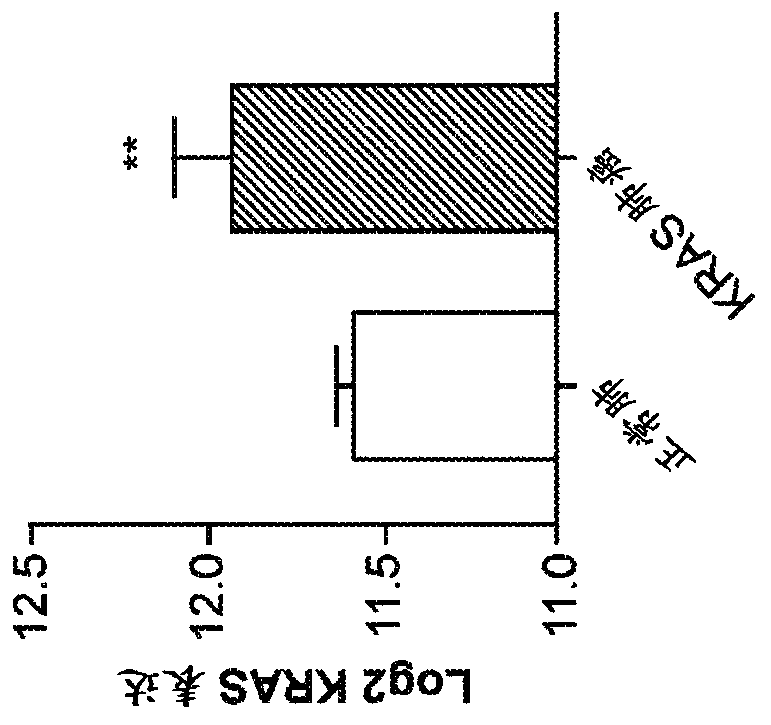
[0150] KRAS activates fatty acid synthase leading to specific ERK and lipid signatures associated with lung adenocarcinoma
[0151] Mutations in the KRAS gene cause lung adenocarcinoma. KRAS activation has been associated with altered glucose and glutamine metabolism. Here, KRAS is shown to activate adipogenesis, and this produces distinct proteome and lipid profiles. By gene expression analysis, KRAS was shown to be associated with lipogenic gene signature and specific induction of fatty acid synthase (FASN). Specific changes in lipogenesis and specific lipids were identified by desorption electrospray ionization mass spectrometry imaging (DESI-MSI). By nanoimmunoassay (NIA), KRAS was found to activate the protein ERK2, whereas ERK1 activation was found in non-KRAS-associated human lung tumors. Inhibition of FASN by cerulenin, a small molecule antibiotic, blocked cell proliferation in KRAS-associated lung cancer cells. Thus, KRAS is associated with the activation of ERK2,...
Embodiment 2
[0183] Nanoscale protein measurements can be used to assess intratumor and intrapatient heterogeneity in solid tumors. A nanoimmunoassay (NIA) was used to analyze ERK signaling in fine needle aspiration (FNA) from kidney cancer patients. 39 patients each had 2-3 areas of their kidney tumor sampled by FNA. Such as Figure 15 Indicated in , each circle is tumor FNA averaged across technical replicates (N=91 FNAs). Samples from each patient are connected by vertical lines. Patients are sorted by mean within ERK2 samples. Within isotypes, variation within technical replicates had a mean standard deviation of 1%.
[0184] Among samples from different regions of the same tumor, the variation between samples had a mean standard deviation of 6%. In contrast, standard deviations of proportions measured across different patients ranged from 5% to 22% across isotypes.
[0185] These data suggest that for ERK, a key signaling protein, intra-tumor heterogeneity (6%) is less than intr...
Embodiment 3
[0187] NIA can be used to measure protein in clinical samples from patients with renal cell carcinoma (RCC) and lung cancer
[0188] As described here, NIA charge separation has been used to measure novel proteins in many sample types and in different malignancies. For example, sample types include, but are not limited to, snap frozen tumors, frozen sections embedded in OTC, fine needle aspirate, bone marrow, blood, CTCs, and plasma. Human tumor clinical samples include, but are not limited to, lymphoma, CML, MDS, kidney cancer, lung cancer, and head and neck cancer. Drugs used include, but are not limited to, Atorvastatin, Rigosertide, FTS, and anti-EPHA3. The data presented herein demonstrate that NIA can be used for diagnosis. For kidney cancer, glutaminase levels were measured. For lung cancer, the RAS is distinguished + with RAS - . Furthermore, different types of cancers such as kidney, head and neck cancers are distinguished from each other. The presented data de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com