A combination formulation comprising hmg-coa reductase inhibitor and calcium channel blocker
A composite preparation and composition technology, applied in the field of oral composite preparations, can solve problems such as poor stability, undisclosed amlodipine and rosuvastatin, etc., achieve improved stability, excellent stability and dissolution, and realize manufacturing simple craftsmanship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0056] Experimental Example 1: Interaction between Rosuvastatin Calcium and Amlodipine Besylate
[0057] In order to judge whether it is easy to develop a compound preparation of rosuvastatin calcium and amlodipine besylate, a compounding test in the case where the two raw materials exist alone and in which the two drugs are mixed is carried out.
[0058] Specifically, as a raw material for rosuvastatin calcium (R), a raw material for amlodipine besylate (A) was prepared. A complex in which each raw material, rosuvastatin calcium, and amlodipine besylate was mixed at a weight ratio of 1:1 was prepared (see Table 1 below).
[0059] 【Table 1】
[0060]
[0061]
[0062] (○: no change, ●: change)
experiment example 1-1
[0063] Experimental example 1-1. Confirmation of trait changes under severe conditions
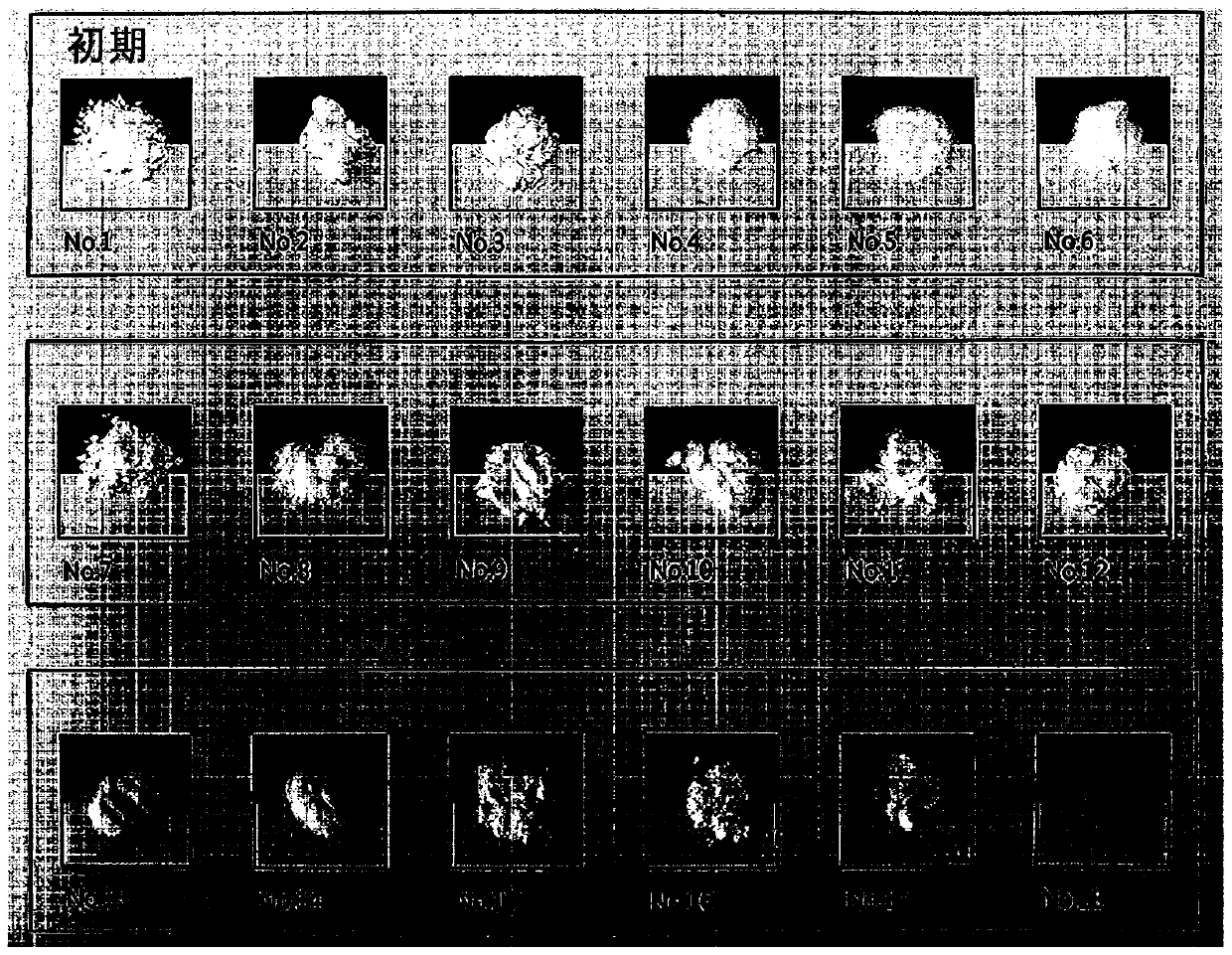
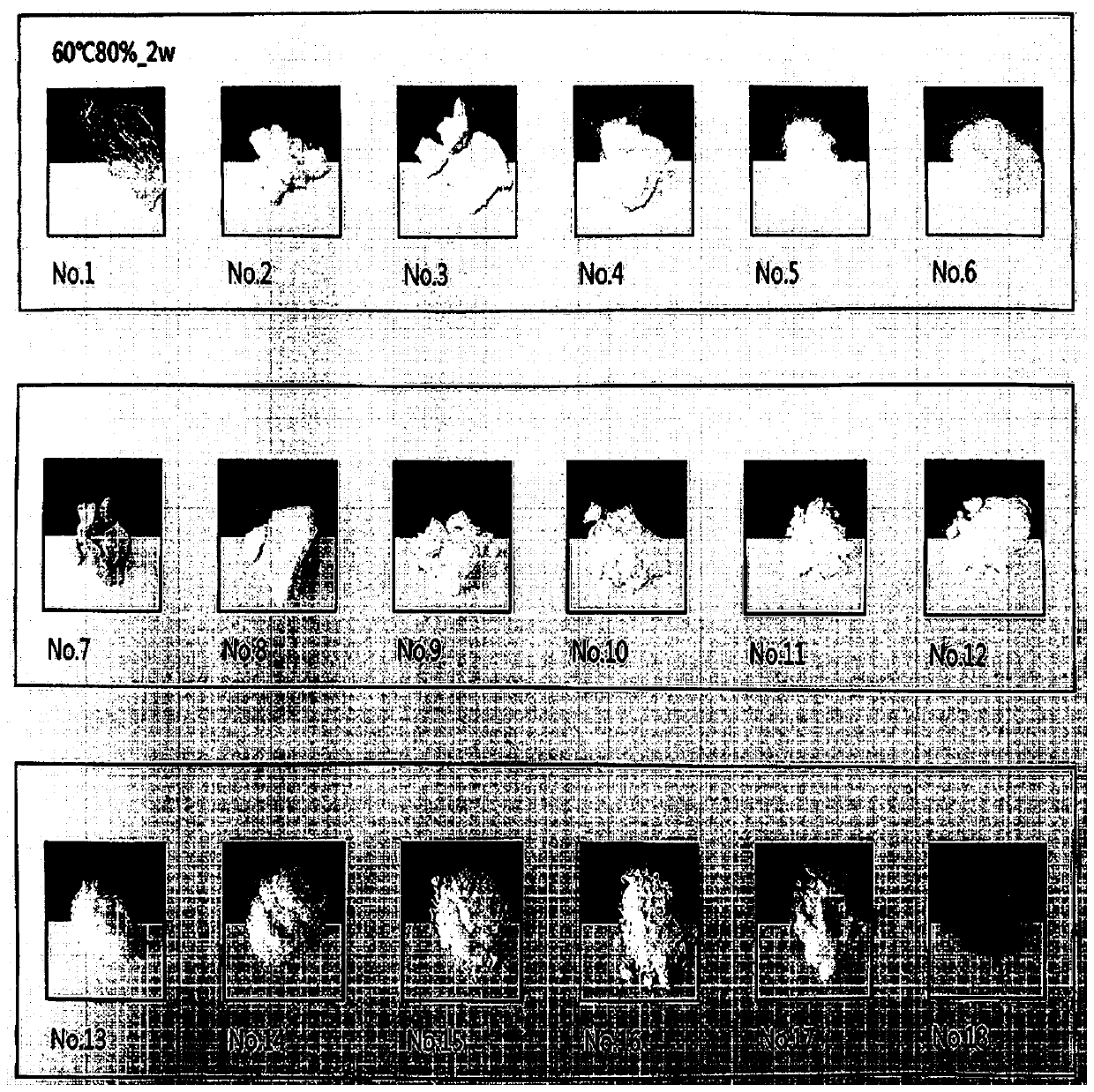
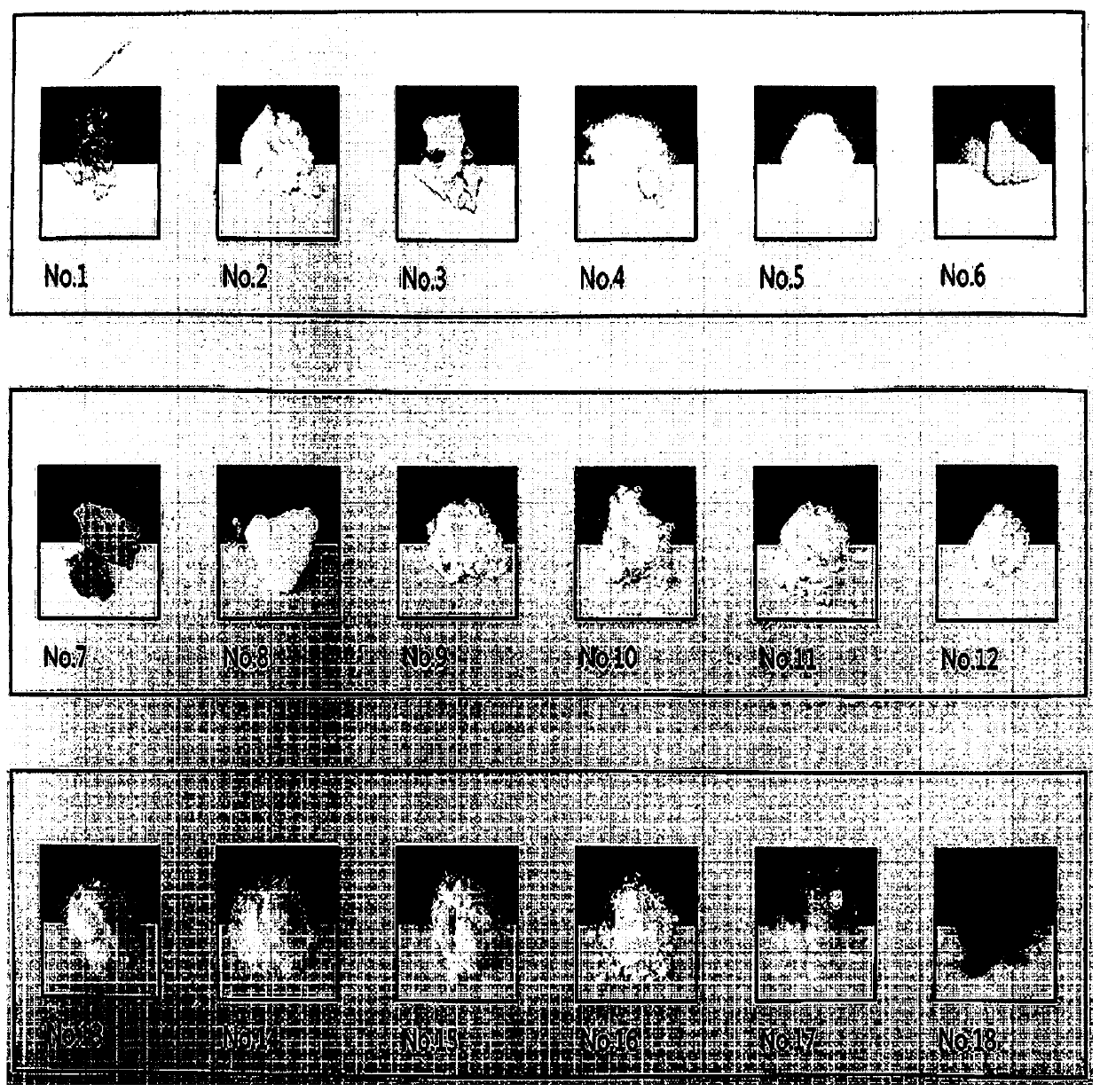
[0064] Under severe conditions, changes in properties of each raw material and complex were confirmed. Specifically, the material was stored at a temperature of 60° C. for 4 weeks, and its properties at the initial stage, after 2 weeks, and after 4 weeks were confirmed.
[0065] Such as Figures 1a to 1c As shown, it was confirmed that amlodipine besylate did not change greatly in properties, but rosuvastatin calcium was solidified and turned yellow. On the other hand, in the case of a compound of two materials, hardening and discoloration were confirmed when cured.
experiment example 1-2
[0066] Experimental example 1-2. Confirmation of content changes of raw materials under severe conditions
[0067] Under severe conditions, changes in the raw material content of each raw material and complex were confirmed. Stored under the same conditions as in Experimental Example 1-1, the change in content was calculated in % by weight and described in Table 2 below.
[0068] 【Table 2】
[0069]
[0070]
[0071] (○: the content is reduced to less than 5%, ● the content is reduced to more than 5%)
[0072] As mentioned above, in the case of amlodipine besylate, no decrease in the level occurred, whereas in the case of rosuvastatin, the level decreased by about 2% at 2 and 4 weeks. On the one hand, in the case of the complex of the two materials, the content of rosuvastatin was reduced by 28% by weight and that of amlodipine by 17% by weight at week 4. From the results, it can be seen that when rosuvastatin calcium and amlodipine contact each other, they have the prop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com