Preparation method of high-purity nitrendipine
A technology for purity of nitren and dipine, which is applied in the field of preparation of high-purity nitrendipine, can solve problems such as non-conformance, difficult to control impurity content, and increased impurity content, and achieves the effect of solving the continuous increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
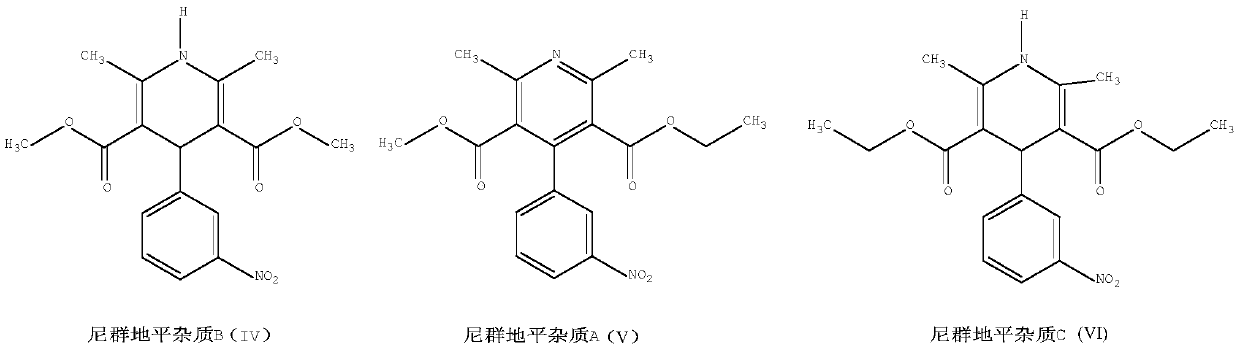
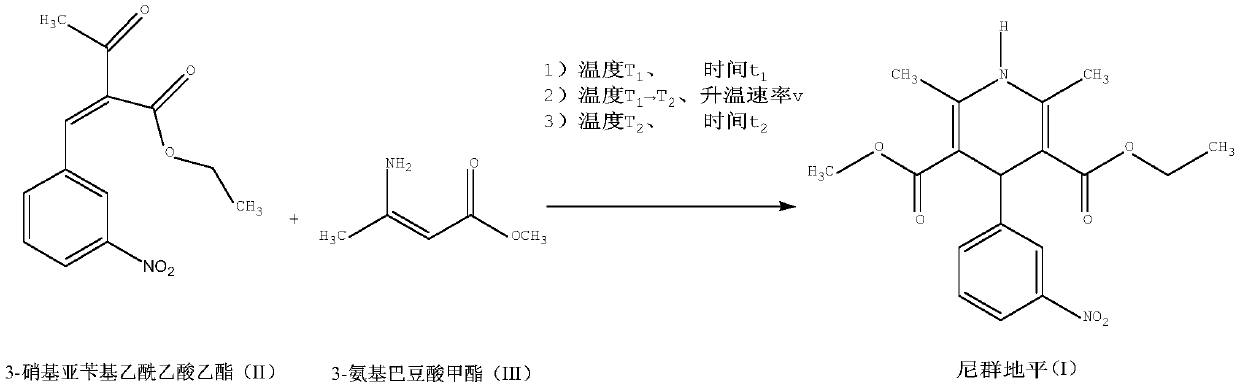
[0032] Add 2.63g (10mmol) of ethyl 3-nitrobenzylidene acetoacetate, 1.15g (10.0mmol) of methyl 3-aminocrotonate, and 2ml of methanol, and heat up to 40°C for 10min. Evaporate the solvent under reduced pressure, and control the heating rate of 0.5°C / min to raise the temperature. When the temperature rises to 90°C, the reaction solution solidifies; react at 90°C for 10 minutes, and immediately lower the temperature; the yellow solid in the reaction bottle is the crude product of nitrendipine, with a weight of 3.61g. 98.41%, yield 98.68%; add 8ml of ethanol, heat up to dissolve, cool down to crystallize, filter at room temperature, 3.05g of nitrendipine fine product, total yield 84.72%, HPLC content 99.71%, impurity B0.18%, impurity A0. 042%, impurity C0.031%.
Embodiment 2
[0034] Add 2.63g (10mmol) ethyl 3-nitrobenzylidene acetoacetate, 1.27g (11.0mmol) methyl 3-aminocrotonate, 8ml ethanol, raise the temperature to 60°C and react for 120min; evaporate the solvent under reduced pressure, and control the temperature rise The temperature was raised at a speed of 2°C / min, and the reaction liquid solidified when the temperature was raised to 110°C; the reaction was carried out at 110°C for 60 minutes, and the temperature was immediately lowered. The yellow solid in the reaction bottle is the crude product of nitrendipine, with a weight of 3.65g, a content of 98.33%, and a yield of 99.69%; 40ml of isopropanol is added, dissolved by heating, cooled to crystallize, and filtered at room temperature to obtain 3.03g of the refined product of nitrendipine. The total yield is 84.16%, the HPLC content is 99.70%, the impurity B is 0.19%, the impurity A is 0.045%, and the impurity C is 0.028%.
Embodiment 3
[0036] Add 2.63g (10mmol) ethyl 3-nitrobenzylidene acetoacetate, 1.21g (10.5mmol) methyl 3-aminocrotonate, 5ml acetonitrile, heat up to 50°C and react for 60min; evaporate the solvent under reduced pressure, and control the temperature rise Heat up at a rate of 1°C / min, and the reaction solution solidifies when the temperature rises to 100°C; react at 100°C for 40 minutes, and immediately cool down; the yellow solid in the reaction bottle is the crude product of nitrendipine, with a weight of 3.58g, a content of 98.49%, and a yield of 97.93%; Add 24ml of acetonitrile, heat up to dissolve, cool down to crystallize, filter at room temperature, and get 3.08g of Nitrendipine fine product, the total yield is 85.55%, the HPLC content is 99.74%, the impurity B is 0.16%, the impurity A is 0.049%, and the impurity C is 0.033%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com