Taccalonolide microtubule stabilizers
A technology of hydroxyl and compound, applied in the field of arrowroot diosconolactone microtubule stabilizer, can solve problems that need to be elucidated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
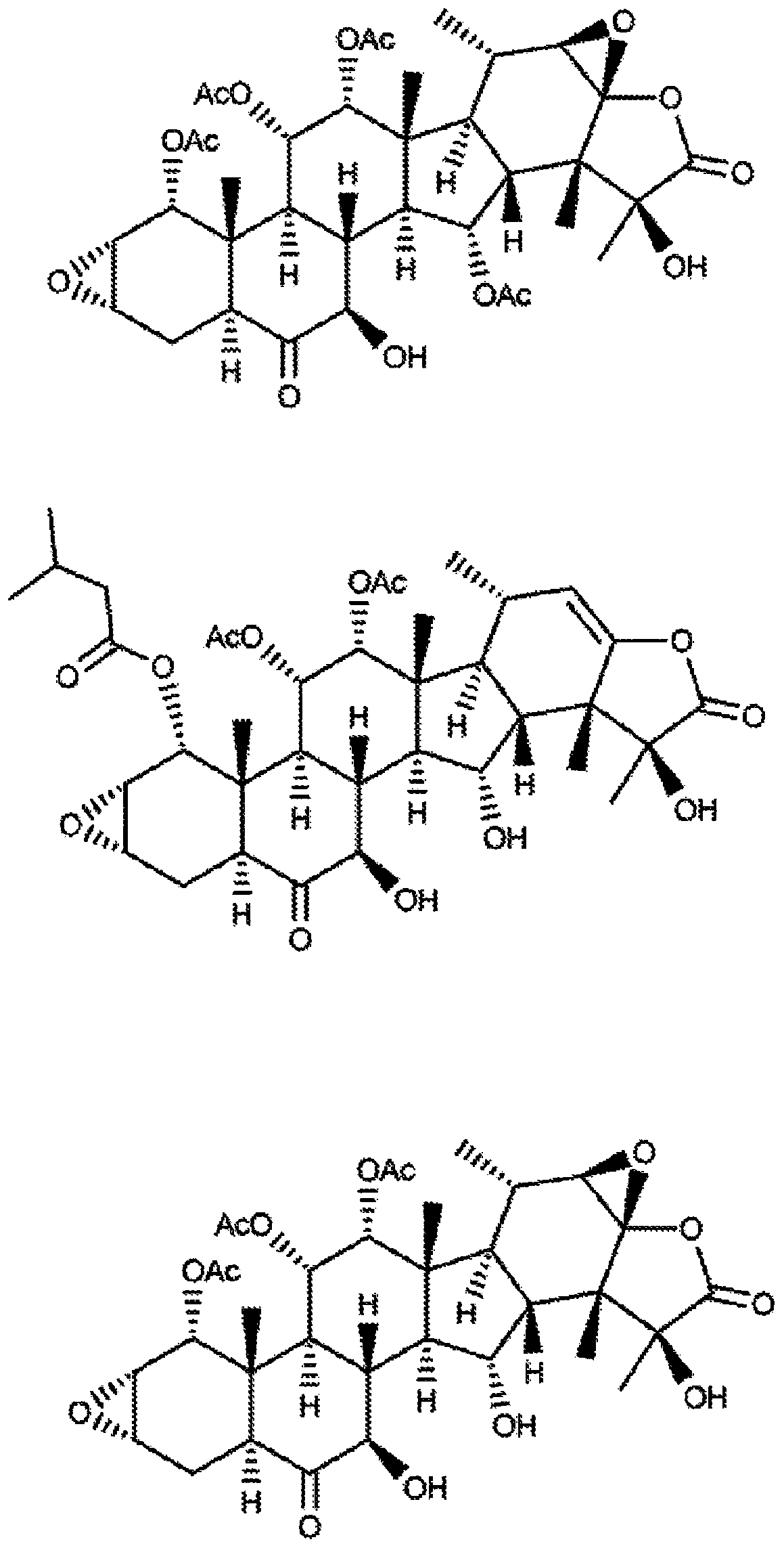
[0067] Arrown diostrogenolides are a unique class of microtubule stabilizers that are active against drug-resistant cells in vitro and in vivo. In the work described below, the present inventors generated novel diostrolides, including diostrolides AF, AJ and AI-epoxides, by isolation and semisynthesis.
[0068] The dioscolide structure was determined by 1D and 2D NMR methods. Each of these dioscolides stabilizes cellular microtubules, leading to the formation of microtubule bundles and mitotic accumulation in cancer cells with multiple aberrant mitotic spindles. IC 50 Values range from the low nanomolar range of diostenolactone AI-epoxide (0.73 nM) and diostonolactone AJ (4.3 nM) to the low micromolar range of diostenolactone R (13 μM) . These studies demonstrate that a variety of diosconolides have microtubule-stabilizing properties and significant structure-activity relationships. These and other aspects of the invention are discussed further below.
[0069] Arrowroot...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap