Triketone compound, preparation method and application thereof, and herbicide
A compound and technology of triketones, applied in the field of triketones and their preparation, herbicides containing the triketones, can solve the problem of lack of HPPD-inhibiting herbicides, and achieve high safety and good control effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
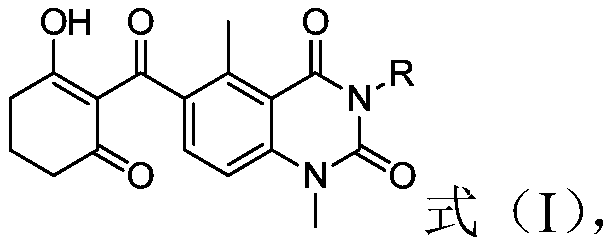
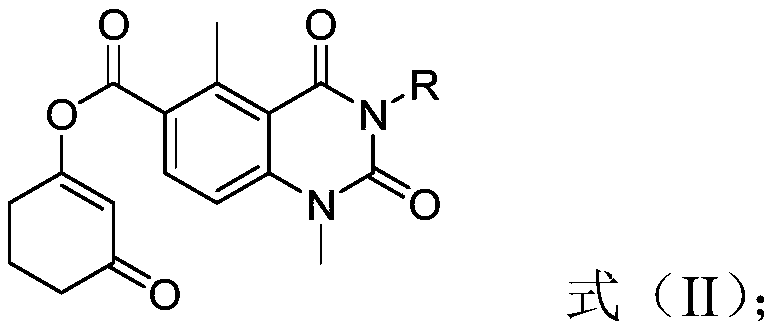
[0035] According to the method for preparing the triketone compound of the structure shown in the formula (I) of the present invention, those skilled in the art can mix the compound of the structure shown in the formula (II) and the catalyst in the base according to the conventional conditions and operation of the rearrangement reaction. Contact is carried out in the presence of a solvent.
[0036] Preferably, the molar ratio of the compound of the structure shown in the formula (II) to the catalyst and the base is 1: (0.01-1): (0.5-4); more preferably, the compound of the structure shown in the formula (II) and the catalyst The molar ratio to base is 1:(0.05-1):(1-3).
[0037] Preferably, the contact conditions include: the reaction temperature is 0-100°C; the reaction time is 0.5-24h; more preferably, the contact conditions include: the reaction temperature is 20-40°C; the reaction time is 5- 12h.
[0038] It should be understood by those skilled in the art that the method...
preparation example
[0059]
[0060] Add 500mmol of the compound shown in 1-1 into a 1L reaction flask at room temperature, add 300mL of glacial acetic acid while stirring, then dissolve 500mmol of ICl into 200mL of glacial acetic acid, and dropwise add it to the above reaction system within 30min while stirring , After the dropwise addition, continue to stir the reaction for about 2.5h. After the reaction was completed, the reaction solution was suction-filtered under reduced pressure, and the obtained solid was washed with 200 mL of acetonitrile and 200 mL of glacial acetic acid, and dried to obtain intermediate 1-2 with a yield of 95%; melting point: 186-188°C. 1 H NMR (600MHz, DMSO-d 6 ): δ8.97 (brs, 3H), 7.72 (d, J = 8.4Hz, 1H), 6.75 (d, J = 7.8Hz, 1H), 2.40 (s, 3H).
[0061] Add 30mmol of intermediate 1-2 into a 100mL two-neck flask, add 60mL of pyridine, and slowly add 30mmol of the compound shown in 1-3 into the system while stirring. The reaction liquid was heated to 100° C. to react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com